Saumya Agrawal1, Amit Gupta1, Sweety Gupta2, Bela Goyal3, Rohik Anjum T. Siddeek1, Deepak Rajput1, Udit Chauhan4, Sanjeev Kishore5, Manoj Gupta2, Ravi Kant1
1 Department of Surgery, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
2 Department of Radiation Oncology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
3 Department of Biochemistry, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
4 Department of Radiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
5 Department of Pathology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
| Date of Submission | 09-Apr-2020 |
| Date of Decision | 17-Apr-2020 |
| Date of Acceptance | 26-Apr-2020 |
| Date of Web Publication | 27-Jun-2020 |
Correspondence Address:
Amit Gupta
Department of Surgery, All India Institute of Medical Sciences, Rishikesh – 249 203, Uttarakhand
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_10_20
Abstract
INTRODUCTION: Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) have been reported in previous studies to assess the prognosis of gall bladder cancer (GBC) individually and in combination. However, the evidence of utility of preoperative CA 19-9, CEA and carbohydrate antigen 125
( CA 125) in determining the resectability and prognosis of GBC is still lacking. In the present study we correlated the serum levels of tumor markers CA 19-9, CEA and CA 125 individually and combined to determine the resectability and prognosis of the GBC.
MATERIALS AND METHODS: Seventy one diagnosed patients of GBC between January 2018 and September 2019 were included in the present study. Serum CA 19-9, CEA and CA 125 were determined by chemiluminescence. Receiver operating characteristic (ROC) curve was used to evaluate the role of tumor markers in determining the resectability of GBC. The Kaplan Meier survival curves were made and log rank analysis was performed to assess the prognostic role of tumor markers in terms of overall median survival.
RESULTS: All the three tumor markers CA19-9, CEA and CA 125 showed high discriminatory power in determining the resectability with respective area under curve of 0.76, 0.68 and 0.78 as determined by ROC. Median survival in patients with high serum CA 19-9, CA 125 was significantly lower than patients with normal serum CA 19-9, CA 125 whereas no significant difference was observed in case of CEA.
CONCLUSION: The present study suggested that CA 19-9, CEA and CA 125 can predict resectability in GBC and raised levels of CA 19-9 and CA 125 can predict poor prognosis in patients with elevated levels.
Keywords: Gall bladder cancer, resection, survival, tumor markers
| How to cite this article: Agrawal S, Gupta A, Gupta S, Goyal B, Siddeek RA, Rajput D, Chauhan U, Kishore S, Gupta M, Kant R. Role of carbohydrate antigen 19-9, carcinoembryonic antigen, and carbohydrate antigen 125 as the predictors of resectability and survival in the patients of Carcinoma Gall Bladder. J Carcinog 2020;19:4 |
| How to cite this URL: Agrawal S, Gupta A, Gupta S, Goyal B, Siddeek RA, Rajput D, Chauhan U, Kishore S, Gupta M, Kant R. Role of carbohydrate antigen 19-9, carcinoembryonic antigen, and carbohydrate antigen 125 as the predictors of resectability and survival in the patients of Carcinoma Gall Bladder. J Carcinog [serial online] 2020 [cited 2021 Oct 15];19:4. Available from: https://carcinogenesis.com/text.asp?2020/19/1/4/288191 |
Introduction
Carcinoma gall bladder is the most common malignancy of the biliary tract, usually presents in advanced stages due to the lack of any typical clinical features and only about 10% of them are the candidates for curative surgery.[1],[2] No screening test or definitive tumor marker for the diagnosis or prognosis is yet available. However, certain tumor markers such as carbohydrate antigen (CA) 19-9, carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 125 are associated with hepatobiliary pathologies, including both benign and malignant ones.[3],[5] These markers, when combined with clinical and imaging findings may guide further plan of the management. The mainstay of treatment of gall bladder cancer (GBC) remains radical resection. It is estimated that 75% of the cases of GBC are already unsuitable for resection in non-incidentally diagnosed patients. Preoperative imaging, available facilities and surgeon’s expertise are the determining factors. However, not all patients have a resectable disease at the time of diagnosis, as in cases of metastases and involvement of major vascular structures by the tumor. Furthermore, some patients turned out unresectable intraoperatively. Small peritoneal and liver metastases <1 cm may be missed on the conventional contrast enhanced computer tomography (CECT) of the abdomen that is used for staging and may lead to unnecessary surgical planning and general anaesthesia for the patient. Preoperative serum tumor markers CEA, CA125, and CA19-9 have been found to improve the prediction of resectability in other hepatobiliary malignancies like cholangiocarcinoma[6] and CA 19.9 has been shown to predict pancreatic cancer resectability.[7] Whereas, to the best of our knowledge none of the previous study has utilized serum tumor markers in assessing resectability in GBC. Previous studies have reported role of Serum CEA or CA19-9 in assessing the prognosis of GBC individually. However, there are few reports providing detailed evaluation of value of combining these two or three tumor markers in predicting the prognosis of patients with GBC. However, evidence of combination of three tumor markers CA 19-9, CEA and CA 125 in determining prognosis in GBC is still lacking. In the present study we analyzed the serum levels of tumor markers CA 19-9, CEA and CA 125 individually and in combination to determine the resectability and prognosis in GBC.
Materials and Methods
This prospective observational study was conducted between January 2018 and September 2019 after obtaining approval from Institutional Ethics Committee (Reference No: AIIMS/IEC/18/177). Informed consent was taken from all patients. Seventy one patients aged ≥18 years, clinically and radiologically suspected or histopathologically biopsy or fine needle aspiration cytology (FNAC) proven cases of gall bladder carcinoma were included in the present study. Patients having synchronous or metachronous malignancy or pregnancy were excluded. Complete history and clinical examination was done. CECT abdomen and pelvis were done as per the standard protocol to stage the disease and staging was done as per the American Joint Committee on Cancer (AJCC) eighth staging system. The resectability was determined based on the radiological investigations. The involvement of hepatic artery, portal vein, direct extension into adjacent organs, enlarged aorto caval lymph nodes, presence of omental or peritoneal deposits, all of which deemed the patients unresectable. If resectable, resection of gallbladder and dissection of regional lymph nodes were performed and wedge resection of the liver with 2 cm margin (including segments IVb/V) was done and the specimen was sent for histopathological examination. If unresectable, ultrasound guided FNAC/biopsy was done, following which the patient received chemotherapy.
Five milliliters venous blood was collected after obtaining informed consent in plain vacutainer with clot activator (BD vacutainers, USA). Blood was centrifuged and serum separated within 2 hours of collection and investigated with serum CA19-9, CEA, CA-125 levels by chemiluminescence on ADVIA Centaur® XP system (Siemens Healthineers; Germany). The samples were run only after satisfactory level of performance by two levels of internal quality controls (low and high) as per manufacturer’s instructions. The ADVIA Centaur CA 19-9 assay is a two-step sandwich immunoassay using direct chemiluminometric technology which uses a single monoclonal antibody, 1116-NS-19-9, for both the solid phase and lite reagent. The antibody is covalently coupled to the paramagnetic particles in the solid phase and the same clone of antibody is labeled with acridinium ester in the lite reagent. The sample and solid phase were incubated at 37°C for 7.5 minutes followed by a wash step to remove excess unbound antigens. The lite reagent was then reacted with solid phase-bound CA 19-9 antigens for an additional 20 min incubation, whereas CEA and CA 125 are a two-site sandwich immunoassay using direct chemiluminometric technology, which uses constant amounts of two antibodies. In case of CEA, first antibody, in the lite reagent, is a purified polyclonal rabbit anti-CEA and rabbit antibody labeled with acridinium ester. The second antibody, in the solid phase, is a monoclonal mouse anti-CEA antibody covalently coupled to the paramagnetic particles. In case of CA 125, the first antibody is directed toward the M11 antigenic domain, and is labeled with acridinium ester. The second antibody is directed toward the OC 125 antigenic domain and was labeled with fluorescein. The immunocomplex formed with CA 125 was captured with monoclonal mouse anti-fluorescein antibody coupled to paramagnetic particles in the solid phase. The cut off levels taken for CA 19-9, CEA and CA 125 were 30.9 IU/ml, 5 ng/ml and 30.2 units/ml respectively, based on the specific kits used for the analysis.
Follow up
All patients were followed up for a period of minimum 12 months after accrual. The endpoint was overall survival (OS) from date of diagnosis and death or the last follow-up. Patients who were lost to follow up were excluded from survival analysis.
Statistical analysis
Patients who were clinically or histopathologically diagnosed with carcinoma gall bladder were grouped into resectable and unresectable and their serum levels of tumor markers CA 19-9, CEA and CA 125 were compared using the Mann- Whitney U test. Receiver operating characteristic (ROC) curve was plotted to calculate area under curve (AUC) to find out discriminatory potential of tumor markers individually and combined in determining resectability of the tumor. P <0.05 was considered statistically significant.
Kaplan-Meier survival curves were drawn for the patients with high and normal levels of three tumor markers taking cut offs of 30.9 IU/ml, 5 ng/ml and 30.2 units/ml (based on the specific kits used for analysis) for CA 19-9, CEA, and CA 125 respectively and their median survival in each group was obtained. Log rank analysis was done to determine the statistical significance of the difference in the survival of the two groups.
Results
This study included 71 patients of histopathologically proven patients of carcinoma gall bladder. Patients included in the study had a mean age of 55.6 years (range 30-80 years). Sociodemographic details of participants are mentioned in [Table 1]. None of the patients had a family history of any cancer. Forty one patients (57.7%) out of 71 had associated gall stones. Abdominal lymphadenopathy (celiac, periportal, perihepatic, peripancreatic, aortocaval and superior mesenteric artery) was detected in 53 patients (74.6%).
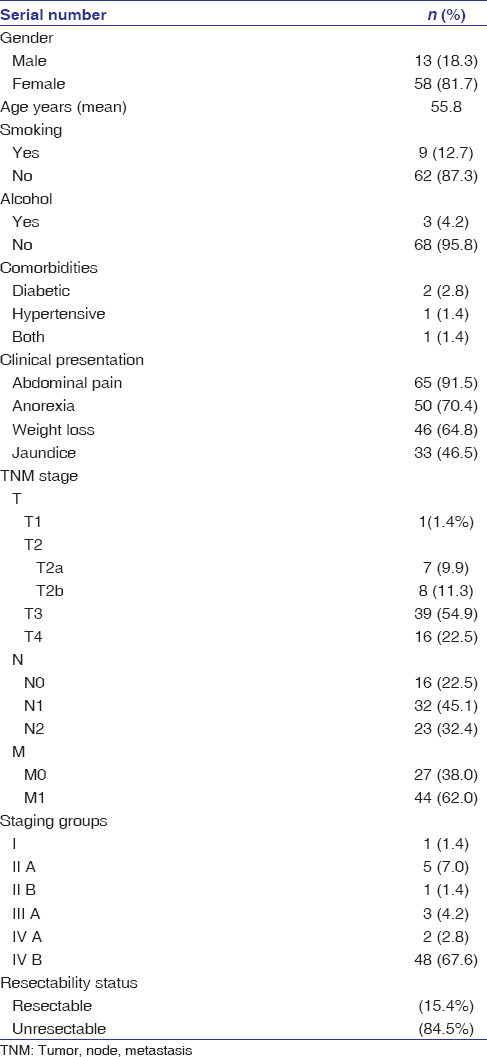 |
Table 1: Sociodemographic profile of patients of carcinoma gall bladder Click here to view |
Omental/peritoneal/liver deposits were reported in 44 patients (62%). Based on clinical and radiological findings, disease was staged as per the AJCC eighth edition.
Carbohydrate antigen 19-9, carcinoembryonic antigen, carbohydrate antigen 125 and resectability of gall bladder cancer
Fifteen out of 71 patients were planned for the surgery based on imaging but a preoperative, staging laparoscopy deemed 2 patients unresectable due to metastases which were not seen on computed tomography (CT) scan whereas two patients had significant lymphadenopathy, which was again not identifiable on CT scan. Therefore, fourpatients were unresectable and only biopsy from the gall bladder lesion was taken. Hence, only 11 out of 71 patients (15.5%) patients could undergo curative resection. Serum levels of CA 19-9 and CA 125 were significantly higher in unresectable GBC patients as compared to resectable GBC patients. Although serum CEA levels were also high in unresectable cases, the difference was not statistically significant. Median levels of tumor markers in both the groups are shown in [Table 2] and the levels of are shown in scatter plots [Figure 1]a, [Figure 1]b, [Figure 1]c All the three tumor markers CA19-9, CEA and CA 125 showed high discriminatory power in determining resectability with respective (AUC) of 0.76 (95% confidence interval [CI]: 0.584 – 0.937), 0.68 (95%CI: 0.503 – 0.850) and 0.78 (95%CI: 0.612 – 0.947) as determined by ROC Plot. CA19.9, CEA and CA 125 showed sensitivity of 85%, 56.7%, 73.3% and specificity of 72.7%, 81.5% and 81.8% respectively. CA19.9, CEA and CA 125 positive predictive values were 94%, 94.4% and 9.6% and negative predictive values were 25%, 25.7% and 36% respectively. CEA showed sensitivity of 56.7% and specificity of 81.5% at optimal cut-off of 5.57 ng/ml and CA 125 showed sensitivity of 73.3% and specificity of 81.8% at optimal cut-off of 33.95 units/ml. Furthermore, when the three markers were used together, the AUC of 0.73 (95%CI: 0.635 – 0.826) was obtained, which again revealed the potentially good discriminatory power of these markers for resectability. ROC plots for each of the three markers used individually and when combined are shown in [Figure 2]a, [Figure 2]b, [Figure 2]c, [Figure 2]d.
| Table 2: Comparison of serum tumor markers among resectable and unresectable gall bladder cancer Click here to view |
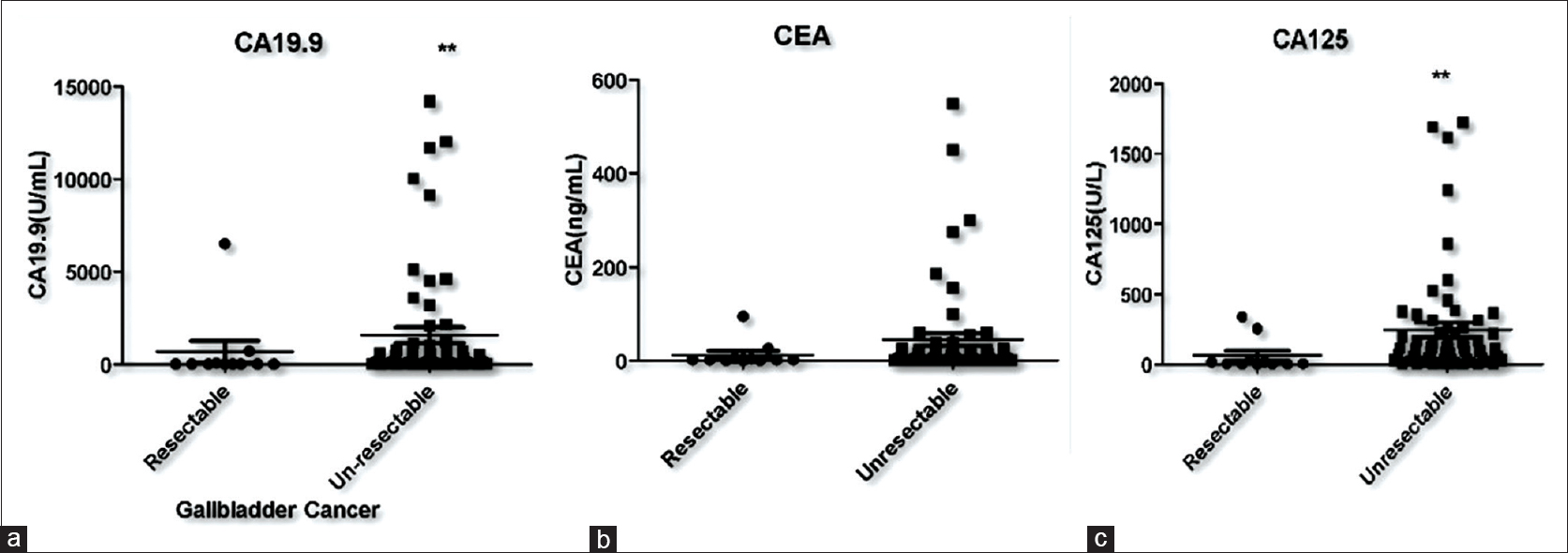 |
Figure 1:Scatter plots demonstrating serum tumor markers levels in resectable and unresectable gall bladder cancer patients (a) carcinoembryonic antigen 19-9 (b) carcinoembryonic antigen (c) carbohydrate antigen 125 Click here to view |
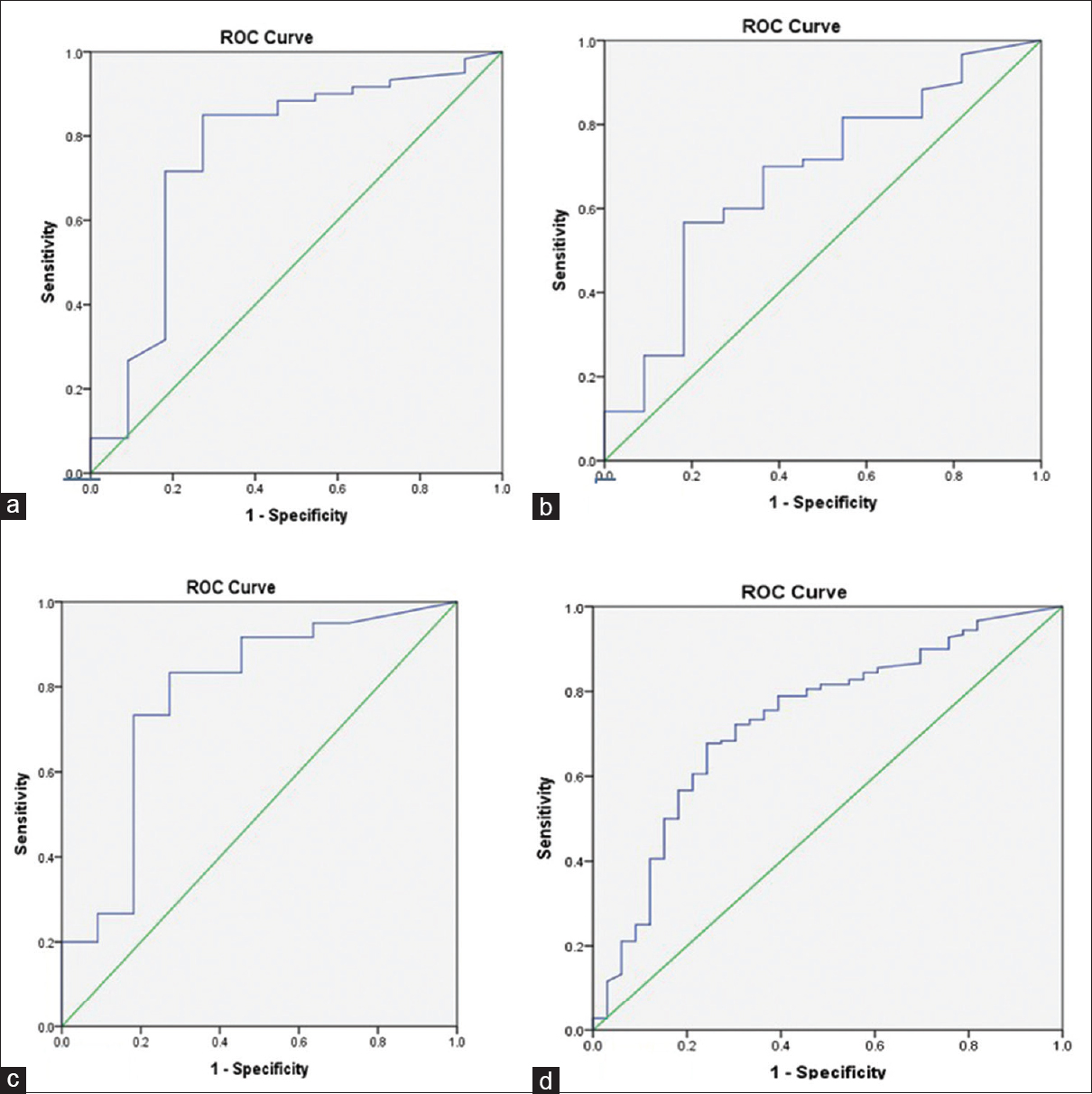 |
Figure 2:Receiver operating characteristic plots demonstrating discriminatory ability of tumor markers in identifying unresectable gall bladder cancer cases ([a] carbohydrate antigen 19-9 area under curve: 0.76; [b] carcinoembryonic antigen area under curve-0.68; [c] carbohydrate antigen 125-0.78; [d] combined three markers-0.7) Click here to view |
Carbohydrate antigen 19-9, carcinoembryonic antigen, and carbohydrate antigen 125 as prognostic markers for gall bladder cancer
Patients were followed for at least 12 months and the survival curves of those with high levels of tumor markers were compared to those with normal serum levels of the markers to assess the prognostic value of these tumor markers in GBC. However, we lost nine patients to follow up, hence only 62 out of 71 could be followed (attrition rate-12.6%). Log rank (Mantel-Cox test) was applied to determine the significance of the difference. The analysis showed that patients with high levels of serum CA 19-9, CEA and CA 125 had lower mean survival than patients with below normal level of tumor marker survival curves have been shown in [Figure 3]a, [Figure 3]b, [Figure 3]c.
| Figure 3:Kaplan–Meier survival curves of the tumor markers (a) carbohydrate antigen 19-9 (b) carcinoembryonic antigen, (c) carbohydrate antigen 125 of gall bladder cancer patients Click here to view |
Median survival in patients with high serum CA 19-9, CA 125 were significantly lower than patients with normal serum CA 19-9, CA 125 whereas no significant difference was observed in case of CEA. Patients with high levels of CA 19-9 had a median survival of 2 months while those with normal levels had the median survival of 5 months. The difference was statistically significant with P 0.03. Although the median survival of patients with high serum CEA levels was only 2.5 months as compared to 4 months of those with low serum CEA levels, the difference was not found to be statistically significant, with P 0.10. The patients with high levels of CA 125 had a median survival of 2 months while those with normal values had a median survival of 7 months. This difference was found to be statistically significant with P 0.0015.
Discussion
Tumor markers occur in blood or tissue and are produced by tumor associated with a cancer or by the host in response to cancer and its is useful for clinical diagnosis or patient management. These markers can be used for screening, diagnosis and monitoring of response. CEA is an oncofetal antigen, associated with plasma membrane of tumor cells. It is raised in colon, lung, gastric and breast cancer. Furthermore it is raised in some benign conditions such as pancreatitis, cirrhosis and inflammatory bowel disease. CA 19-9 is a CA raised in gastric cancer, lung cancer, colon cancer and pancreatic cancer. CA 125 is a carbohydrate related glycoprotein raised in non- mucinous ovarian tumors, lung, endometrial, pancreas, breast, and colon and non-malignant conditions for example menstruation, pregnancy and endometriosis.[8] Tumor markers CEA, CA 19-9, CA-125 CA-242 have been used in many cancers for example liver, gastric, colorectal, pancreas for diagnosis, prognosis and for detecting recurrences.[9],[10] These markers have also been used to predict resectability in cholangiocarcinoma, pancreatic cancers and gastrointestinal cancers.[11] These markers have been used individually for the diagnosis of GBC, but the results have been inconsistent and better sensitivity was observed when used in combination.[12],[13],[14]
Previous studies have assessed the effect of serum levels of tumor markers (AFP, CEA, CA19-9, CA72-4, TPA and TK), on prognosis of carcinoma gall bladder and found that TK, TPS and CEA were the independent prognostic factors forOS in GBC.[15],[16],[17]
Furthermore in one study neutrophil to lymphocyte ratio and CEA were associated with poor OS of GBC patients.[16] Lee = al assessed CEA and CA 19-9 levels post chemotherapy and found CA 19-9 as the independent and most valuable prognosticator.[17] Similar findings have been reported by Yu et al., who studied CA 19-9 and CEA as the independent prognostic markers in resectable GBC.[18] In contradiction Agarwal et al. identified that with raised serum CA 19-9 and CA 242 levels median survival were less but was not statistically significant.[19] In the present study along with CEA and CA19-9, serum CA 125 levels were also evaluated as the prognostic marker to predict survival in GBC and similar to previous studies higher levels of tumor markers were found to be associated with poor overall median survival whereas contrary to previous studies CEA did not show a significant effect on the survival of GBC patients. Fang et al. reported that preoperative CEA, CA125, and CA19-9 were associated with resectable cholangiocarcinoma whereas higher levels were associated with unresectable tumor.[6] Similar to our study they have also shown lower AUC with CEA due to its nonspecific nature. Similarly studies have suggested varying role of CA 19-9, CEA and CA125 in predicticting resectability of hilar cholangiocarcinoma[20] and pancreatic cancer.[21] The present study for the first time evaluated the relation of resectability of GBC with the serum levels of tumor markers and found statistically significant difference in the serum levels of CA 19-9 and CA 125. The same holds true when these two tumor markers are combined, however no such statistical difference was found in serum CEA levels.
Conclusion
To conclude tumor markers CA 19-9, CEA and CA 125 may predict resectability and OS of GBC patients in adjunct to other radiological investigations with reasonable sensitivity and specificity. However, the results need to be confirmed through a study with larger study population, especially those in early stages of GBC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. | |
| 20. | |
| 21. |

