Roopesh Poojary1, Nayanatara Arun Kumar1, Reshma Kumarchandra2, Ganesh Sanjeev3, D Shivananda Pai4, NA Vinodini1, K Bhagyalakshmi1
1 Department of Physiology, Kasturba Medical College Mangalore, Manipal Academy of Higher Education, Manipal, Karnataka, India
2 Department of Biochemistry, Kasturba Medical College Mangalore, Manipal Academy of Higher Education, Manipal, Karnataka, India
3 Microtron Centre, Department of Studies in Physics, Mangalore University, Mangalagangotri, Karnataka, India
4 Department of Neurology, Kasturba Medical College Mangalore, Manipal Academy of Higher Education, Manipal, Karnataka, India
| Date of Submission | 07-Oct-2019 |
| Date of Decision | 28-Feb-2020 |
| Date of Acceptance | 28-Mar-2020 |
| Date of Web Publication | 27-Jun-2020 |
Correspondence Address:
Nayanatara Arun Kumar
Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Karnataka
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_13_19
Abstract
INTRODUCTION: Radiation is an important tool in the diagnostic and curative treatment of many cancers. Ionizing radiation induces many biochemical changes in the cells. The present study was designed to estimate the level of neurotransmitters in the distinct brain tissue of Swiss albino mice before exposing gamma radiation.
MATERIALS AND METHODS: The mice were treated with 0.25 and 1 g/kg body weight of Cynodon dactylon extract (CDE) via oral gavage for 7 days and subjected to 5 Gy of gamma radiation. The estimation of monoamines was performed in the cortex and cerebellum separately.
RESULTS: Mice exposed to a sublethal dose 5 Gy of gamma radiation causes a significant decrease in dopamine, norepinephrine, epinephrine, and serotonin levels compared to normal. The mice treated with 0.25 and 1 g/kg body weight of CDE via oral gavage for 7 days showed significant improvement in the level of monoamine neurotransmitters in both the cortex and cerebellum homogenate.
CONCLUSION: Oral administration of antioxidant-rich C. dactylon has shown a neuromodulatory effect against radiation-induced depletion of neurotransmitters in the brain tissues.
Keywords: Cerebellum, cortex, Cynodon dactylon, gamma radiation, neurotransmitters, oxidative stress
| How to cite this article: Poojary R, Kumar NA, Kumarchandra R, Sanjeev G, Shivananda Pai D, Vinodini N A, Bhagyalakshmi K. Assessment of monoamine neurotransmitters in the cortex and cerebellum of gamma-irradiated mice: A neuromodulatory role of Cynodon dactylon. J Carcinog 2020;19:6 |
| How to cite this URL: Poojary R, Kumar NA, Kumarchandra R, Sanjeev G, Shivananda Pai D, Vinodini N A, Bhagyalakshmi K. Assessment of monoamine neurotransmitters in the cortex and cerebellum of gamma-irradiated mice: A neuromodulatory role of Cynodon dactylon. J Carcinog [serial online] 2020 [cited 2021 Oct 15];19:6. Available from: https://carcinogenesis.com/text.asp?2020/19/1/6/288193 |
Introduction
Exposure to radiation has become an inevitable process of human life in this modern era. It may be natural sources or artificial sources such as medical diagnostic, therapeutic procedures, space studies, nuclear energy or weapon testing sites, and nuclear accidents. In the clinical aspect, radiations are usually utilized in diagnostic and therapeutic purposes. There are many different medical conditions where ionizing radiations are critical in clinical applications and its involvement in different levels of exposure of radiation during the procedure. However, the disadvantage is that ionizing radiation generates free radicals and causes damage to vital molecular components.[1] Therefore, any approach that has a potent role in protecting the lethal effect of radiation without conceding the antitumor activity is of great clinical significance.[2] Synthetic radioprotective agents possess various side effects. Development of effective, inexpensive, and nontoxic radioprotective agents still remains as an active area of research. Therefore, a finding with the help of a natural agent preventing the cells from free radical mediated damage is of prime importance.[3] Neurotransmitters are the sole chemicals that change the synaptic activity and thereby neuronal function. Abnormality in the levels of neurotransmitters leads to most of the nervous diseases and mental disorders.[4] The neurotransmitters such as dopamine, norepinephrine, epinephrine, and serotonin play a very important role in various neurological functions. All these four neurotransmitters are amino acid-derived neurotransmitters.[5] Dopamine generates inhibitory postsynaptic potentials and plays an potent role in the movement, learning, and motivation.[6] Neurons that contain the epinephrine-forming enzyme have their cell bodies in the brainstem regions in the rat brain and send projections mainly into other brainstem areas, hypothalamus, and spinal cord.[7] Serotonin is found in many tissues such as the brain, inside the lining of the digestive tract and blood platelets. It has an excitatory on pathways that are involved in the control of muscles and an inhibitory effect on pathways that mediate sensation. In the brain, serotonin has also been concerned in sleep, regulation of body temperature, mood, depression, and anxiety.[8] Alterations in these neurotransmitters in the brain can be well correlated with brain dysfunctions. The regional difference of sensitivities of monoamines in the distinct brain tissues during irradiation has not been explored yet.[9]
Cynodon dactylon belongs to family Poaceae, commonly known as Bermuda grass, Couch Grass, Devil’s Grass, and Indian Doab. It is a hard and perennial grass native of the warm temperate and tropical regions. This is a commonly available herb that has been reported numerous antioxidant compounds. Literature survey documents the anticancer, antiulcer, anti-inflammatory, antidiabetic, antimicrobial, and DNA protective role in various animal models.[10] The diversity and richness of antioxidant compounds present in the C. dactylon extracts (CDEs) documented from our earlier studies provoked us to explore the protective role of this extract on dopamine, norepinephrine, epinephrine, and serotonin levels in important brain areas, such as cortex and cerebellum.[11],[12],[13]
Materials and Methods
Ethics
All the procedures for the animal experiments were reviewed and approved by the Institutional Animal Ethical Committee at Kasturba Medical College, Mangalore (F. No. 25/439/2009; approved date: March 31, 2016). All animal studies were conducted and maintained according to the guidelines proposed by the Committee for Control and Supervision of Experimentation on Animals (Reg. No. 213/CPCSEA), Government of India.
Animals
Adult Swiss albino male mice which are 6–8 weeks old weighing 250 ± 2 g were used for all in vivo studies; all the mice used for the experimentation procedures were obtained from the Central Animal House, Kasturba Medical College, Mangalore. Throughout the research period, animals were maintained at a temperature of 25°C ± 2°C and a humidity of 50% ± 5% with 12 h of dark-light cycles. Further, mice were provided with ad libitum access to laboratory food (commercial mice pellets from VRK Nutritional Solutions, India) and water.
Radiation facility
Mice were placed in well-ventilated circular Perspex box restrainers. Six mice were placed in single restrainers and exposed to whole-body radiation of gamma rays. The source of radiation was low-dose gamma irradiation (11 Gy/min) from the Centre for Application of Radioisotopes and Radiation Technology, Mangalore University, Mangalagangotri, Karnataka, India.
Plant collection and extract preparation
C. dactylon (both aerial and root part) was collected from nearby area Kasturba Medical College, Manipal Academy of Higher Education. The taxonomical identification and authentication were done by a taxonomist. Plants were washed under tap water, shade dried, and powdered. The extract was prepared by 50:50 ratio of methanol in water and refluxed at 50°C–60°C in a Soxhlet apparatus for 72 h. The liquid output was cooled and concentrated by rotary vacuum flash evaporator. The hydroalcoholic extract was kept in sterile bottle, until further use.
Acute drug toxicity of hydroalcoholic extract of Cynodon dactylon
Acute toxicity studies of hydroalcoholic extract of C. dactylon was done, and it was nontoxic up to 5 g/kg body weight. It did not display any symptoms of behavioral changes and mortality up to 14 days’ observation period. Therefore, 1/20th and 1/5th of this dose, i.e., 0.25 and 1 g/kg body weight, were used as low dose and high dose, respectively, in the subsequent study.
Mice grouping
Mice were randomly divided into six groups with 12 mice in each group with 12 mice in each group (total 72 mice) as follows:
- Control group (G-1): Normal healthy mice receiving distilled water via oral gavages for 7 days
- Radiation control group (G-2): Mice exposed to 5 Gy of gamma radiation
- Low-dose CDE group (G-3): Mice received a low dose of CDE at 0.25 g/kg body weight for 7 days
- High-dose CDE group (G-4): Mice received a high dose of CDE at 1 g/kg body weight for 7 days
- Low-dose CDE + Irradiated group (G-5): Mice administered with 0.25 g/kg body weight of CDE for 7 days, and the last dose of extract was given orally hour before exposure to 5 Gy of gamma rays
- High-dose CDE + Irradiated group (G-6): Mice received 1 g/kg body weight of CDE for 7 days, and the last dose of extract was given orally hour before exposure to 5 Gy of gamma rays.
All the mice were sacrificed at 24-h postirradiation by cervical dislocation. The whole brains were removed quickly and washed using chilled saline. The blood and other debris were removed from the tissue.
Sample preparation
The cerebellum and cortex were separated out from the whole brain. The tissue homogenates (10%) were prepared in ice-cold phosphate-buffered saline (pH 7.4). The homogenates were centrifuged and the supernatant was collected separately.
Analysis of dopamine, norepinephrine, epinephrine, and serotonin level by an enzyme-linked immunosorbent assay
The sandwich enzyme-linked immunosorbent assay kits are precoated with capture primary antibody, i.e., mouse neurotransmitter monoclonal antibody. Standards/samples are added to antibody precoated wells followed by incubation. Postincubation, antirespective neurotransmitter antibody labeled with biotin and streptavidin-HRP are added to plate, resulting in the formation of immune complex. Unbound enzymes removed by washing the plate after incubation substrates were added to the plates. The solution will turn blue and change to yellow with the effect of acid. The shades of solution and the concentration of mouse neurotransmitters are positively correlated. Neurotransmitter levels were quantified using commercially available kit following the manufacturers’ protocol [Companies are listed in [Table 1].
| Table 1: List of companies of enzyme-linked immunosorbent assay kits used for estimation Click here to view |
Statistical data analysis
All data were expressed as mean ± standard error of the mean. Data were analyzed using one-way analysis of variance following Tukey’s post hoc test using IBM SPSS Statistics for Windows, Version 20.0 Armonk, NY: IBM Corp. P <0.05 was considered statistically significant.
Results
Dopamine level
Irradiated group (G-2) showed a significant decline in dopamine levels of the cortex and cerebellum when compared to normal control group (G-1). In the mice pretreated with CDE, a significant decline in the dopamine level was observed in the cortex (G-5, P = 0.049; G-6, P = 0.005) and cerebellum (G-5, P = 0.050; G-6, P = 0.015) homogenates when compared to radiation control group (G-2) [Figure 1].
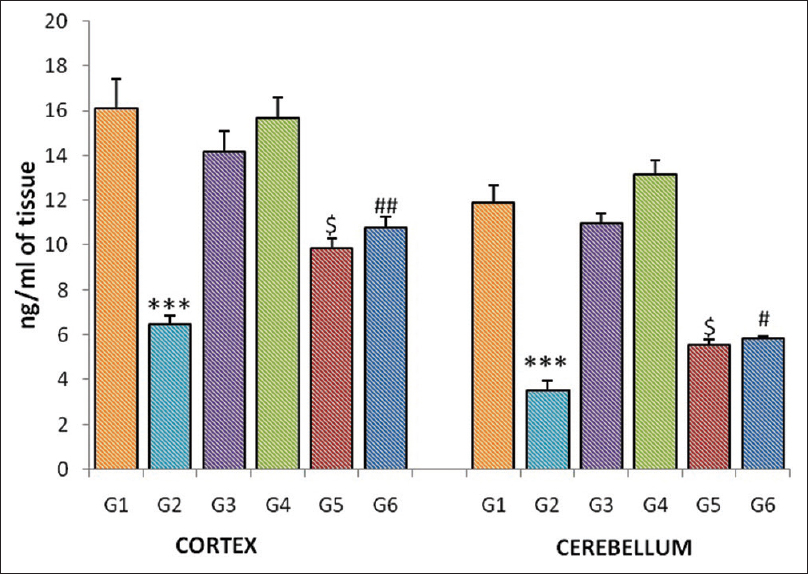 |
Figure 1: Dopamine level of the cortex and cerebellum in different groups of mice. Each bar represents mean of dopamine ± standard error of the mean, n = 12. For comparison with Group 1 and Group 2, the significant levels, *** P <0.001; for comparison with Group 2 and Group 5, the significant levels,$P <0.05; for comparison with Group 2 and Group 6, the significant levels,#P <0.05,##P <0.01 Click here to view |
Norepinephrine level
Gamma-irradiated group (G-2) showed a significant reduction (P <0001) in the norepinephrine level in the homogenates of the cortex and cerebellum when compared to normal control group (G-1). Further, a significant increase in the cortex was observed in the pretreated CDE groups (G-5, P = 0.026; G-6, P = 0.002) when compared to radiation group (G-1). Norepinephrine level was also significantly increased in the cerebellar tissue in the low and high doses of CDE-pretreated groups (G-5, P = 0.028; G-6, P = 0.002) [Figure 2].
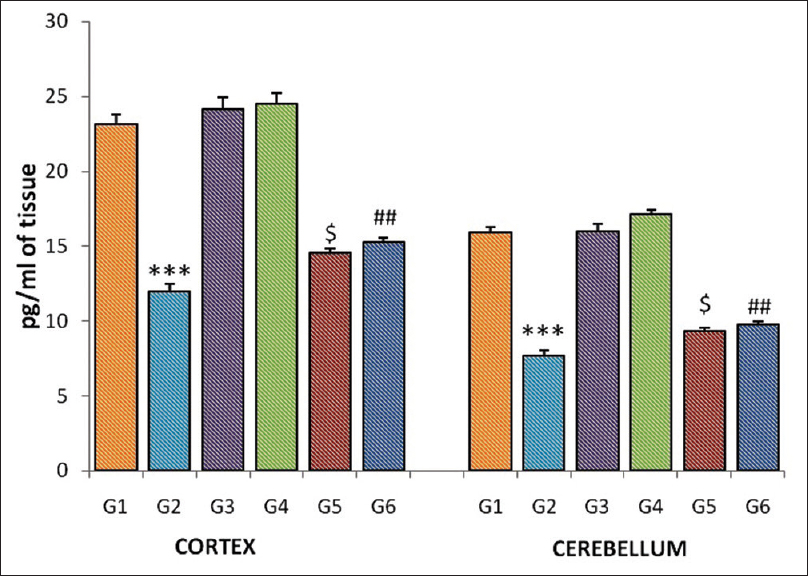 |
Figure 2: Norepinephrine level of the cortex and cerebellum in different groups of mice. Each bar represents mean of norepinephrine ± standard error of the mean, n = 12. For comparison with Group 1 and Group 2, the significant levels, *** P <0.001; for comparison with Group 2 and Group 5, the significant levels,$ P <0.05; for comparison with Group 2 and Group 6, the significant levels,## P <0.01 Click here to view |
Epinephrine level
The significant reduction (P <0001) in the epinephrine level was observed in the homogenates of the cortex and cerebellum in the gamma-irradiated group (G-2) when compared to normal control (G-1). Pretreatment of CDE significantly increased the epinephrine level in the cerebellum (G-5, P = 0.023; G-6, P = 0.013) and cortex homogenates (G-5, P = 0.023; G-6 P = 0.004) when compared to the radiation control group (G-2) [Figure 3].
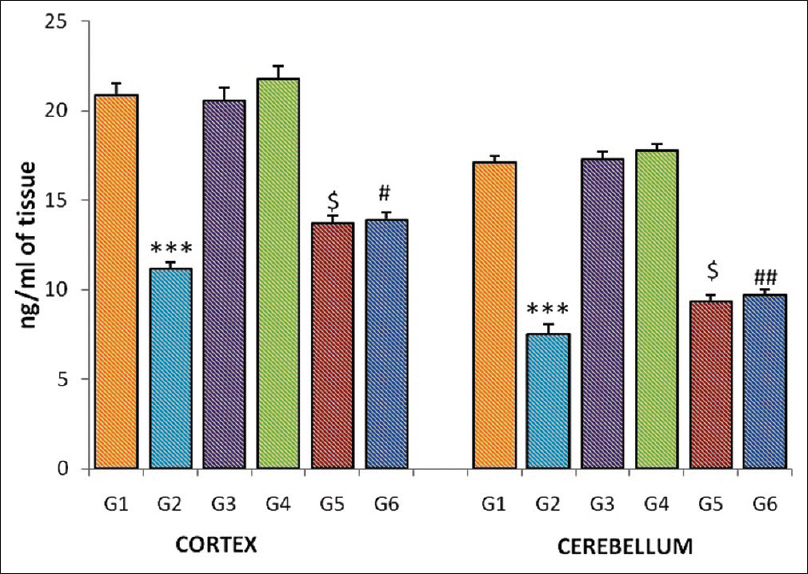 |
Figure 3: Epinephrine level of the cortex and cerebellum in different groups of mice. Each bar represents mean of epinephrine ± standard error of the mean, n = 12. For comparison with Group 1 and Group 2, the significant levels, *** P <0.001; for comparison with Group 2 and Group 5, the significant levels,$ P <0.05; for comparison with Group 2 and Group 6, the significant levels,# P <0.05,## P <0.01 Click here to view |
Serotonin level
Homogenates of the cortex and cerebellum showed a significant reduction (P <0.0001) in the serotonin level in the gamma-irradiated group (G-2) when compared to normal control (G-1). The serotonin level was significantly increased in the cortex in pretreated CDE groups (G-5, P = 0.027; G-6, P = 0.000) when compared to radiation group (G-1). The epinephrine level in the cerebellar tissue also showed a significantly increase in low and high dose of CDE-pretreated groups (G-5, P = 0.025; G-6, P = 0.003) when compared to radiation control group (G-2) [Figure 4].
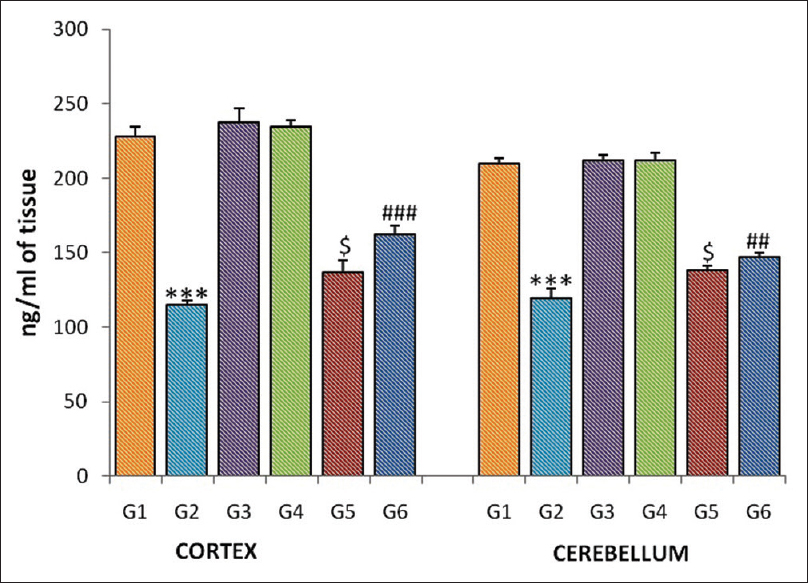 |
Figure 4: Serotonin level of the cortex and cerebellum in different groups of mice. Each bar represents mean of serotonin ± standard error of the mean, n = 12. For comparison with Group 1 and Group 2, the significant levels, *** P <0.001; for comparison with Group 2 and Group 5, the significant levels,$ P <0.05; for comparison with Group 2 and Group 6, the significant levels,## P <0.01,### P <0.001 Click here to view |
The changes in level of dopamine, norepinephrine, epinephrine, and serotonin in the homogenates of the cortex of different groups are concluded in [Table 2], and the changes in the level of dopamine, norepinephrine, epinephrine, and serotonin in the homogenates of the cerebellum of different groups are concluded in [Table 3].
| Table 2: Changes in the level of dopamine, norepinephrine, epinephrine and serotonin in homogenates of the cortex of different groups Click here to view |
| Table 3: Changes in the level of dopamine, norepinephrine, epinephrine and serotonin in homogenates of the cerebellum of different groups Click here to view |
Discussion
Screening for active radioprotective agents is of prime interest in view of their potential application for the advantage of humankind during intentional and unintentional radiation exposures. Even though several synthetic agents were projected as effective radioprotectors, their usage was limited due to their side effects.[14] The present study demonstrates the phytotherapeutic significance of C. dactylon as an effective radioprotective agent in the distinct brain tissues. Till date, no study has explored the radioprotective potential of C. dactylon against radiation-induced neurotransmitter alterations in the cerebral cortex and cerebellum.
The generation of oxidative stress in the cells results when the ionizing radiation creates an imbalance in the existing antioxidant and pro-oxidant condition.[15] Among all the other organs, brain is highly vulnerable to oxidative damage due to its high lipid content and poor endogenous antioxidant mechanisms.[16] Recovery of damage through cell division cycle cannot happen properly because of postmitotic condition neurons.[17] The elevated level of reactive oxygen species (ROS) produces the defect in the structural and functional mechanisms.[18] One of the possible reflections depicting the extent of brain damage is through the variations in the brain neurotransmitter system.[19] The serotonergic and the noradrenergic systems appear to be the good possible source to analyze the extent of damage due to its wide distribution in the brain tissues.[20] All these neurotransmitters play a critical role in various brain functions. The changes in the levels of these neurotransmitters have been well associated with various neurodegenerative diseases.[21] The present study results showed that sublethal 5 Gy doses of gamma radiation produced significant deprivation in the level of biogenic amines in the cortex and cerebellum. This suggests the defects in molecules involved in the dopaminergic system and the serotonergic system and its association with the significant induction of oxidative.[22] Decreased level of the neurotransmitters observed in this study might be attributed to decreased synthesis and reduced absorption, resulting from radiation-induced damage.[23] The observed decline in the neurotransmitter level might be due to the reduction of precursors. Decrease in the absorption of tryptophan would reduce the synthesis of serotonin, while a decrease in the absorption of L-tyrosine may diminish the production of dopamine, norepinephrine, and epinephrine.[24],[25] Experimental evidence also supports the various biochemical and functional alterations in the brain after the exposure to ionizing radiations. Radiation-induced alteration in the metabolism of these monoamines might be one of the reasons, leading to brain dysfunction.[26] The present results suggest the strong association between different biogenic amines and production of ROS in the radiation-induced rat brain. Unlike synthetic drugs, natural antioxidants can perform multiple site-specific targets without causing toxicity to host body.[27] The potent ability of these phytochemicals able to cross the blood–brain barrier could be attributed to the role as so neuroprotectors.[28] In our study, mice pretreated with hydroalcoholic extract C. dactylon for 7 days through orally in two different doses showed increase in the neurotransmitter level of both in the cortex and cerebellum. The pretreatment of the plant-based compounds in animal models has shown the improvement endogenous enzymes such as catalase, superoxide dismutase, and glutathione peroxidase during oxidative stress.[29] Our previous studies documented the presence of phytochemical constituents in CDE such as phenolic acids, flavonoids, tannins, steroids, saponins, glycosides, and alkaloids. Further, bioactive polyphenolic flavonoids such as gallic acid, orientin, rutin, and morin have also been proved by High Perfomance Liquid Chromatography analysis.[11] These compounds are the major natural antioxidants which are the potential ROS scavengers. Dose-dependent increase in all activities of antioxidant content, flavonoid, ABTS scavenging assay, hydroxyl radical scavenging, total phenolic content, iron chelating, reducing potential, and DPPH scavenging has also been documented in our previous study reports.[12] Further, pretreatment of CDE for 7 days improved spatial learning and memory and motor coordination along with the improvement of antioxidant enzymes, and decline in oxidative stress markers has also been observed in the cerebellum of mice.[13] Diversity of antioxidant constituent phytocompounds present in the CDE might have exerted the increase in neurotransmitter in the cerebral cortex and cerebellum against radiation-induced damage. The present study reports are in well consistent with the study reports of effect of pomegranate juice in minimizing the neurotransmitter alterations.[30],[31]
Conclusion
The present findings demonstrated the radioprotective potential of C. dactylon in rendering protection against radiation-induced neurotransmitter alterations in the cerebral cortex and cerebellum. The presence of phenolic compounds and antioxidant components might have minimized neurotransmitters’ alteration and oxidative damage caused by radiation exposure. C. dactylon which is nontoxic, cost-effective, antioxidant-rich, and readily available can be used as a neuromodulator in any kind of oxidative stress condition. The diverse antioxidant property of effects of this extract might be useful against various free radicals and nitric oxide-mediated human pathophysiological conditions. Further in-depth studies with positive neurotransmitter inducer might provide more insight in understanding the potent effect of C. dactylon for advanced therapeutic approach.
Acknowledgment
We thank the Board of Research in Nuclear Science (BRNS), Mumbai, of Atomic Energy, Government of India, for providing the funding and we are also thankful to the Manipal Academy of Higher Education for providing all the facilities needed for this research work.
Financial support and sponsorship
This study was funded by the BRNS, Mumbai, Department of Atomic Energy, Government of India (Grant No. 34 (1)/14/38/2014-BRNS/1932/27/11/2014).
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. |
Ashokkumar K, Selvaraj K, Muthukrishnan SD. Cynodon dactylon (L.) Pers.: An updated review of its phytochemistry and pharmacology. J Med Plants Res 2013;7:3477-83. [Internet].2013Dec25[cited2018Oct17]Availablefrom: https://academicjournals.org/journal/JMPR/article-abstract/236954B42438
 |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. | |
| 25. | |
| 26. | |
| 27. | |
| 28. | |
| 29. | |
| 30. | |
| 31. |
