Jennifer Cullen1, Sally Elsamanoudi1, Stephen A Brassell2, Yongmei Chen3, Monica Colombo4, Amita Srivastava5, David G McLeod2
1 Center for Prostate Disease Research, Department of Defense, Rockville; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda; and Department of Surgery, Uniformed Services University of the Health Sciences, Bethesda, MD, USA
2 Center for Prostate Disease Research, Department of Defense, Rockville; Department of Surgery, Uniformed Services University of the Health Sciences, Bethesda; and Walter Reed National Military Medical Center, Bethesda, MD, USA
3 Center for Prostate Disease Research, Department of Defense, Rockville; and Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, MD, USA
4 Department of Surgery, Uniformed Services University of the Health Sciences, Bethesda; and Walter Reed National Military Medical Center, Bethesda, MD, USA
5 Center for Prostate Disease Research, Department of Defense, Rockville, MD, USA
| Date of Submission | 27-Dec-2011 |
| Date of Acceptance | 10-Jan-2012 |
| Date of Web Publication | 19-Mar-2012 |
Correspondence Address:
Jennifer Cullen
Center for Prostate Disease Research, Department of Defense, Rockville; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda; and Department of Surgery, Uniformed Services University of the Health Sciences, Bethesda, MD
USA
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1477-3163.94025
Abstract
Introduction: In this review, the International Agency for Research on Cancer’s cancer epidemiology databases were used to examine prostate cancer (PCa) age-standardized incidence rates (ASIR) in selected Asian nations, including Cancer Incidence in Five Continents (CI5) and GLOBOCAN databases, in an effort to determine whether ASIRs are rising in regions of the world with historically low risk of PCa development. Materials and Methods: Asian nations with adequate data quality were considered for this review. PCa ASIR estimates from CI5 and GLOBOCAN 2008 public use databases were examined in the four eligible countries: China, Japan, Korea and Singapore. Time trends in PCa ASIRs were examined using CI5 Volumes I-IX. Results: While PCa ASIRs remain much lower in the Asian nations examined than in North America, there is a clear trend of increasing PCa ASIRs in the four countries examined. Conclusion: Efforts to systematically collect cancer incidence data in Asian nations must be expanded. Current CI5 data indicate a rise in PCa ASIR in several populous Asian countries. If these rates continue to rise, it is uncertain whether there will be sufficient resources in place, in terms of trained personnel and infrastructure for medical treatment and continuum of care, to handle the increase in PCa patient volume. The recommendation by some experts to initiate PSA screening in Asian nations could compound a resource shortfall. Obtaining accurate estimates of PCa incidence in these countries is critically important for preparing for a potential shift in the public health burden posed by this disease.
Keywords: Age-standardized incidence rate, Asia, cancer incidence in five continents, GLOBOCAN, prostate cancer
How to cite this article:
Cullen J, Elsamanoudi S, Brassell SA, Chen Y, Colombo M, Srivastava A, McLeod DG. The burden of prostate cancer in Asian nations. J Carcinog 2012;11:7
How to cite this URL:
Cullen J, Elsamanoudi S, Brassell SA, Chen Y, Colombo M, Srivastava A, McLeod DG. The burden of prostate cancer in Asian nations. J Carcinog [serial online] 2012 [cited 2021 Oct 14];11:7. Available from: https://carcinogenesis.com/text.asp?2012/11/1/7/94025
Introduction
International statistics on the descriptive epidemiology of prostate cancer (PCa), including incidence and mortality rates, are available from databases maintained by the International Agency for Research on Cancer (IARC), including Cancer Incidence in Five Continents (CI5) and GLOBOCAN. [1],[2],[3],[4],[5],[6],[7],[8],[9],[10],[11] The IARC’s CI5 databases provide the oldest and most accurate estimates of international cancer statistics, spanning over 50 years of data collection. [12] More recently, the IARC launched the GLOBOCAN database that provides estimates on cancer incidence and mortality for a subset of 27 cancer sites from most nations of the world over two reporting periods. The most recent version, GLOBOCAN 2008, has been used for estimating worldwide cancer experience. The CI5 data are reported for a smaller number of nations with data derived from regional or national cancer registries. There are a total of nine CI5 volumes currently available (I-IX), dating back to the first published volume in 1966 up to the most current Volume IX, published in 2007 and reflecting a reporting period from 1998-2002. In contrast, the GLOBOCAN 2008 database includes cancer statistics on 184 countries recognized as independent nations according to the United Nations (UN) World Population Prospects Report. [13] GLOBOCAN 2008 excludes countries with a population of less than 100,000 and does not separate out Hong Kong and Macau from China, as does the UN. While extensive in scope of international coverage, the GLOBOCAN 2008 estimates of cancer incidence and mortality are derived using a variety of methodologies and data sources; whereas CI5 data are derived from 86 cancer registries worldwide, covering 101 populations and 28 cancer sites. In order to directly compare CI5 and GLOBOCAN cancer incidence statistics, one must use the age-standardized incidence rate (ASIR) which accounts for differences in the age structure within each nation or region. [14],[15]
According to the most recent reporting periods, CI5 2002 and GLOBOCAN 2008 demonstrate that the nations with the highest PCa ASIRs include Australia, Austria, New Zealand, and the United States. In contrast, the lowest ranking nations with respect to PCa ASIRs include many of the Asian nations, such as India and China, and Japan. Yet, over the past decade, data from a number of independent studies, along with CI5 data from successive reporting periods, reveal a steady increase in PCa ASIRs in several Asian nations. Underscoring the importance of these trends are recent reports using population-based cancer registries that demonstrate steady, incremental rises in PCa ASIRs over time. [16],[17],[18],[19] Factors that underlie rising PCa incidence estimates might include the dissemination of PSA screening, routine access to medical care, proximity to medical care, education, income, race/ethnicity, and reported behavior (e.g., diet) and genetic predisposition. [20],[21] Ultimately, a complex interplay of these factors may all contribute to PCa development.
A working group on Asian Perspectives on Cancer Control (APCC) was formed to examine patterns in PCa incidence and mortality within Asian nations and to formulate recommendations for cancer screening and treatment [16],[22] with recognition for the need to improve cancer incidence and mortality data collection efforts in Asia. Moreover, the APCC recently called for improving early PCa detection by formal recommendation of prostate-specific antigen (PSA) screening for the first time. [19],[23]
The purpose of this review is to examine one key component of the descriptive epidemiology of PCa in selected Asian nations, the age-standardized incidence rate (ASIR). The IARC CI5 Volumes I-IX will be used as the primary source of ASIR estimates. The CI5 data will be complemented by GLOBOCAN 2008 international data. A secondary goal is to provide PCa ASIR trends over time based on CI5 Volumes I-IX.
Materials and Methods
Selection of countries
The selection criteria for determining which Asian nations would be examined in this review were as follows: using the CI5 Indices of Data Quality for CI5 Volume IX, only nations with a minimum of 80% microscopically verified (%MV) PCa cases were selected, as a metric of data accuracy in cancer case ascertainment. [1] Second, the percent of PCa cases registered from a death certificate, only (%DCO) was considered. [1] Only nations with %DCO of less than 5% were considered. In instances in which multiple registries within a given nation contributed data quality indices, the %MV and %DCO cut points were required of more than 50% of the reporting regions for the nation to be considered for this review. Inadequate data quality based on these cut points led to the exclusion of India, Israel, and the Philippines. Due to concerns of unstable ASIR estimates for nations with low counts of PCa cases, those nations with fewer than 1000 PCa cases for an entire reporting period were excluded. This criterion resulted in the elimination of Kuwait, Malaysia, Oman, Pakistan, and Thailand. Finally, in order to examine change over time, only nations providing ASIR estimates from a minimum of the two most recent CI5 reporting periods (1993-1997 and 1998-2002, respectively) were included. [1,9] This last selection criterion led to the exclusion of Bahrain, Cypress, Turkey and Vietnam.
Because PCa is a disease of aging men, the male life expectancy (LE) in these eligible nations was examined to confirm that each had an average male LE of at least 70 years, according to two data sources: the United Nations (UN) Statistical Yearbook for Asia and the Central Intelligence Agency (CIA) World Fact Book. [13],[24]
This selection process resulted in the following study sample of Asian nations: China, Japan, Republic of Korea (South), and Singapore.
International data sources
Cancer incidence in five continents (CI5)
The CI5 Volumes I-IX public use data were used as the primary data source for international estimates of PCa ASIR data. [2],[3],[4],[6],[7],[8],[9],[10],[11],[13],[24] Using the IARC CI5Plus online analysis tool, PCa ASIR estimates were generated per nation for all years in which data were available. [1],[2],[3],[6],[7],[8],[9],[10],[11] Prostate cancer was classified according to the International Classification of Disease Version 10 (C61). In China, registry data were reported to CI5 2002 from the following regions: Hong Kong; Shanghai; Jiashin; Nangang District, Harbin City; Guangzhou City; and Zhongshan. In Japan, registry data were obtained from CI5 2002 for the following prefectures: Miyagi, Osaka, Nagasaki, Yamagata, Aichi, and Fukui, as well as the city of Hiroshima. For the Republic of Korea, CI5 2002 data were reported from Busan, Daegu, Seoul, Daejeon, Gwangju, Incheon, Jejudo, Ulsan and the Korean Central Cancer Registry. Finally, Singapore data were reported by CI5 2002 at the level of the Chinese, Indian, and Malay populations, as well as for Singapore’s National Cancer Registry. The CI5 data were provided at the level of national region or population-based cancer registry. The ASIR estimates are expressed as the number of new prostate cancer cases per 100,000 person-years. A weighted mean of age-specific rates are standardized to an external population known as the 1960 World Standard Population, to account for difference in the population age structure across reporting regions. The details on data sources and computation of age-standardized incidence rates for CI5 2002 data can be found on the IARC website. [1] The CI5 2002 data represent incidence rates for the calendar years 1998-2002.
Time trends for PCa ASIR estimates from CI5 Volumes I-IX were generated on IARC’s CI5plus website, using the online analysis graphing tool. Among the four options for line charts, “time trends” was chosen. Selections were then made for type of cancer (prostate), gender (male), registry (Asian nation and its associated registries), time period (first and last available start and end dates), statistic (age-standardized incidence rate), and age (0 to 85+). An aggregated statistic representing the ASIR per national registry was then generated using these selections. Data were not available for the Republic of Korea or the Indian population subset of Singapore when using the online CI5 graphing tool.
GLOBOCAN 2008
The GLOBOCAN 2008 PCa ASIR estimates were extracted from the IARC website. [5] Similar to CI5 estimates, the ASIRs represent a weighted mean of age-specific rates, standardized to the World Standard Population. While GLOBOCAN estimates were also published in 2002, the variation in incidence and mortality estimation methods used previously prevents direct comparison of GLOBOCAN 2008 with GLOBOCAN 2002. Therefore, as recommended by IARC, only the most current estimates are reported in this review.
The detailed methodologies and data sources used by GLOBOCAN to estimate cancer statistics for those nations included in its 2008 database are described in detail on the GLOBOCAN website. [5] Estimates from GLOBOCAN 2008 reflect the most recently available data per nation, varying from two to five years previous to the GLOBOCAN 2008 release. The GLOBOCAN 2008 estimates are provided at the level of the nation.
Similar to the method of obtaining CI5 data, GLOBOCAN 2008 public use data were obtained using an online analysis tool to generate PCa ASIRs after selecting cancer type (prostate, ICD-10-C61), age group (default values of 0 to 75+), gender (male), data type (incidence) and continent (Asia).
Results
Overall findings
In all four Asian nations examined, the PCa ASIRs were observed to increase over time, though not linearly. [Table 1] provides a summary of the PCa ASIRs for each nation based on CI5 Volumes I-IX databases. As a criterion for study inclusion, at a minimum all nations were required to have data from the two most recent CI5 reporting periods, 1993-1997 (Volume VIII) and 1998-2002 (Volume IX). However, within a nation, not all reporting regions or registries had data from these two periods.
 |
Table 1: Age-standardized incidence rates (ASIR) of prostate cancer by CI5 volume, for registry regions in selected Asian countries Click here to view |
Within China, Hong Kong and Shanghai had the most complete data, as well as the highest rates observed [Table 1]. Hong Kong experienced a near tripling in PCa ASIRs, rising from 5.1 to 15.0 new PCa cases per 100,000 person-years between 1974-1977 (Volume IV) and 1998-2002 (Volume IX) whereas Shanghai reported a more than eightfold increase from 0.8 to 6.9 new PCa cases per 100,000 person-years across the same time periods.
In Japan, the Miyagi prefecture had the oldest data and the most significant increase over time, rising from 3.8 to 22.0 new PCa cases per 100,000 person-years [Table 1]. Similarly, all other prefectures and Hiroshima experienced increases in ASIRs over time, with the exception of Aichi and Fukui that only reported data for Volume IX so that change over time could not be examined.
In the Republic of Korea, increases in PCa ASIR estimates were noted [Table 1]. However, only for Busan, Daegu, and Seoul were data available from at least two reporting periods, permitting examination of change over time. In all three of these Korean reporting regions, PCa ASIRs were slightly higher in Volume IX versus Volume VIII. The most significant change was observed in Seoul where rates rose from 8.5 to 12.7 new PCa cases per 100,000 person-years.
In Singapore, the Chinese, Indian and Malay populations demonstrated substantial rises in PCa ASIR between 1968-1972 (Volume III) and 1998-2002 (Volume IX). Data from the Singapore National Cancer Registry more closely resembled the estimates from the Chinese and Malay groups, as opposed to the Indian group which skewed lower for all periods examined. An almost five-fold increase from 3.8 to 18.6 new PCa cases per 100,000 person-years was noted in the Chinese population while Malay rose from 4.8 to 16.1 new PCa cases per 100,000 person-years. The Indian rates showed the least consistent pattern, fluctuating in directionality of change over time for the CI5 reporting periods.
In comparing PCa ASIR estimates across CI5 2002 and GLOBOCAN 2008, important differences were noted [Table 2]. The GLOBOCAN ASIR statistics were aggregated per nation as opposed to CI5 data which were reported at the level of the individual registry (i.e., China, Japan, Republic of Korea) or population subgroup (i.e., Singapore). In China, the GLOBOCAN 2008 estimates of 4.3 new PCa cases per 100,000 person-years contrasted from the CI5 registry-level estimates that varied from a low of 2.1 and 2.2 new PCa cases per 100,000 person-years in Nangang District and Zhongshan, respectively, to a high of 15.0 new PCa cases per 100,000 person-years in Hong Kong, China. Similarly, in the Republic of Korea, the summary statistic for PCa ASIR from GLOBOCAN 2008 was 22.4 new PCa cases per 100,000 person-years as compared to a low of 5.8 new PCa cases per 100,000 person-years in Daejeon and a high of 12.7 new PCa cases per 100,000 person-years in Seoul, based on CI5 2002 estimates.
[Figure 1] provides a graphical depiction of PCa ASIRs over time for three of the four Asian nations examined for which CI5 data were available using the IARC online graphing tool. It is important to note that Korean data were not available in the time trend analysis and are, therefore, not depicted in the figure. Moreover, the plot for China depicts two registries (Shanghai and Hong Kong). Similarly, in Japan, data from three registries are plotted over time, including Miyagi, Osaka, and Yamagata. Finally, for the nation of Singapore, data from Chinese and Malay subgroups are plotted but Indian data were not available using the online graphing tool. The plot reveals evidence of increases over time in PCa ASIR estimates in all regions for which data are available, most notable in China and Singapore Chinese populations.
 |
Figure 1: Time trends in Prostate Cancer Age-Standardized Incidence Rates (ASIR)* for Three** Asian Nations using “Cancer Incidence in Five, *The ASIRs are measured as data plotted were limited to those available using the online graphing tool on the IARC website.[1-3,6-11], **Data from the Republic of Korea and Indian Singapore populations were not available using this interactive online graphic tool. Click here to view |
Conclusions
In this review, the descriptive epidemiology of PCa, as estimated by ASIRs, was examined within four Asian nations. The primary data source was IARC’s CI5 databases, considered to provide the most accurate international cancer statistics on a limited number of countries worldwide. These CI5 data were supplemented with IARC’s GLOBOCAN 2008 database. The CI5 2002 data were used as the most recent estimates, while all CI5 volumes were examined for time trends in PCa ASIRs.
An increase in rates was observed in all four Asian nations examined. In China, Japan, and Singapore, meaningful change in the PCa ASIRs was noted. In the Republic of Korea, increases were small. Yet, in 2010, Jung et al., examined the Korea Central Cancer Registry (KCCR) for calendar years 2006 and 2007 and revealed PCa ASIRs of 17.7 and 20.0 new PCa cases per 100,000 person-years, respectively. [25] This is in contrast to the CI5 2002 database estimate of 8.5 new PCa cases per 100,000 person-years from the same registry source (KCCR) for the calendar years 1999-2002.
According to the UN World Population Prospects report, there were 4.1 billion persons residing in Asian nations in 2009; this figure is expected to grow to 6 billion persons by 2050, under the assumption of a constant growth rate. [13] Since increasing age is one of few established risk factors for PCa development, it is noteworthy that the life expectancy of males in several of the largest Asian countries has risen steadily over the past decade, including the nation with the greatest number of persons worldwide, India. [13],[20],[24] While Asian nations have historically reported among the lowest PCa ASIRs, there are an increasing number of reports of rising PCa incidence in countries such as Japan, Republic of Korea, and China. Autopsy studies in Japan have shown that, while biological aggressiveness of PCa may be lower for Japanese men as indicated by cancer size at autopsy in comparison to men in the US, the detection of latent disease indicates that current incidence estimates among Japanese men may represent an underestimate of the actual disease burden. [26] International autopsy studies have shown that despite varying incidence rates of PCa around the world, prevalence of disease increases with age in many nations around the world. [27]
When PCa is detected in many Asian nations, it is typically due to onset of symptoms and advanced stage. [19],[23] Therefore, some have argued for introduction of a PSA screening program, similar to that of the US and other developed nations. This is despite the ongoing controversy over PSA screening in the US, blamed by some as contributing toward over-detection of indolent cancer. PSA introduction in nations without a current, national screening program could have a profound impact on PCa ASIRs in a relatively short period of time. While introduction of PSA screening has been associated with a stage migration toward less advanced cancer and a spike in localized disease, there is debate over the impact of stage migration on reductions in PCa mortality. [28],[29],[30],[31]
The relatively high rate of advanced PCa detection in Asian nations has been blamed on lack of a mass screening effort. [32],[33] There are serious implications of widespread PCa screening using PSA in a nation as large as China, where the sheer volume of newly detected cases could create a significant demand on the healthcare delivery system. Whether this demand could sustain a rapid shift in PCa incidence as occurred in the US, has been questioned. Currently, the most common treatment strategy in China is castration while radical prostatectomy (RP) is reportedly uncommon. [32],[33] In contrast, a Japanese Patterns of Care study recently showed that use of radiation therapy for treatment of PCa – used among Japanese men of older age or with advanced stage disease – may more closely resemble US patterns. [34] In the event of a rapid increase in the volume of newly diagnosed PCa cases, there is some question as to whether adequate medical resources, including trained personnel, would be available. In a 2009 CIA report, 189 nations were ranked according to the percent of the gross domestic product (GDP) spent on health. [24] Among the nations examined in this review, the following rankings were observed: China ranked 148 th place at 4.6%, Japan ranked 40th at 9.3%, the Republic of Korea ranked 90 th at 6.5%, and Singapore ranked 168 at 3.9% of the national GDP spent on health. Yet despite some concerns of national readiness to address a rapid rise in the volume of PCa patients, [35] the APCC and other independent researchers have begun to propose widespread PSA screening in Asian nations, including the second largest, China. [19] It is well documented that the dramatic shift in newly diagnosed early-stage PCa that occurred in the US in the late 1980s and early 1990s in the United States was driven by dissemination of PSA screening of asymptomatic men. [29],[31]
Study considerations
A limitation of examining international estimates and trends in PCa ASIRs is the variation in case recording practices or data coding that may change over time, even among the series of CI5 databases. As indicated in [Table 1], the same set of population-based cancer registries were not represented for all time periods examined by CI5. With respect to GLOBOCAN 2008, while extensive in scope of nations represented, this database uses a variety of methodologies and data sources for estimating cancer incidence across countries, depending on data availability within each nation. This varies from use of national registry data, as the most rigorous source, to assignment of neighboring national statistics, as an example of least rigorous. [5] In addition to CI5 and GLOBOCAN data sources, data were sought from cancer registries in these four nations to further capture the PCa ASIR experience. were sought to determine whether PCa ASIRs in the four nations of interest appear to be increasing. Moreover, estimates of PCa ASIRs are collected from a limited number of registry data sources from nations that are geographically and/or ethnically heterogeneous. Such registries offer a limited number of urban and rural data sources to represent the nation as a whole, which may not adequately represent the true burden of disease. Comparability of GLOBOCAN 2008 data to the CI5 2002 estimates is limited by many factors including variation in data sources and methods for computing ASIRs, as well as the level of reporting: while CI5 data are provided at the level of national region or cancer registry, GLOBOCAN data are reported at the level of the nation using registry and other data sources within the nation [Table 2]. Yet, the heterogeneity in CI5 2002 PCa ASIR estimates across registries/populations within China, Japan, Republic of Korea and Singapore is not captured in the GLOBOCAN summary estimate. This observed variation within nations argues against the use of an aggregated ASIR statistic to represent the PCa burden for these nations.
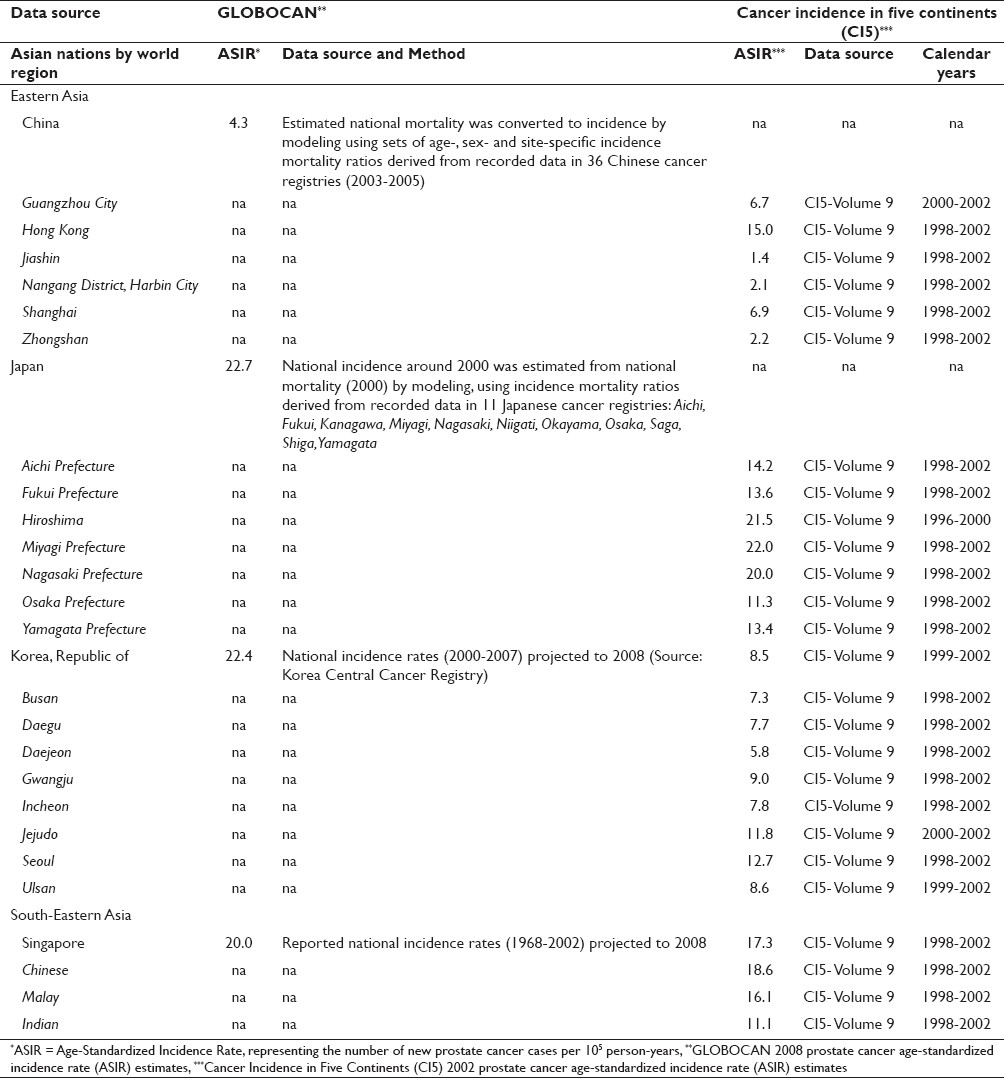 |
Table 2: Comparison of prostate cancer age-standardized incidence rates from two IARC databases Click here to view |
A new standard population was proposed by the World Health Organization in 2001. [36] Though, both CI5 and GLOBOCAN databases continue to use the 1960 World Standard Population (“Segi”), updated by Doll et al., in 1966, as it is still deemed a useful, common metric with which to compare cancer incidence rates across nations. [14],[15],[37]
Finally, while a strict set of selection criteria were used to identify nations to be included in this review, using CI5 data quality indicators, the burden of PCa in other Asian nations deserves attention.
Future directions
The known variability in methods and approaches used to calculate PCa ASIR estimates per data source needs to be considered when attempting to draw comparisons within and across nations. There is a clear need to expand the efforts of population-based cancer registries in Asian countries to better assess the burden of PCa incidence. If there should be an introduction of PSA screening in a large Asian nation such as China, leading to a spike in localized disease as occurred in the US 20 years ago, [31] such a rapid change in disease burden could place demands on the national medical infrastructure which will be difficult to meet over a short time period. [35]
References
| 1. | Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer Incidence in Five Continents: Vol. 9. IARC Scientific Publications No. 160. Lyon, France: International Agency for Research on Cancer; 2007.  |
| 2. | Doll R, Muir CS, Waterhouse JA. Cancer Incidence in Five Continents, Vol. II. Union Internationale Contre le Cancer, Geneva. 1970.  |
| 3. | Doll R, Payne P, Waterhouse JA. Cancer Incidence in Five Continents, Vol. 1. Geneva; Union Internationale Contre le Cancer; 1966.  |
| 4. | Ferlay J, Parkin DM, Curado MP, Bray F, Edwards B, Shin HR, et al. Cancer Incidence in Five Continents, Vol. 1-9. IARC Cancer Base No. 9. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http:// ci0 5.iarc.fr. [Last accessed on 2011 Dec 13].  |
| 5. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Vo1. 2. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10. Lyon, France: International Agency for Research on Cancer. Available from: http://globocan.iarc.fr. [Last accessed on 2011 Jan 11].  |
| 6. | Muir CS, Waterhouse J, Mack T, Powell J, Whelan SL. Cancer Incidence in Five Continents, Vol. 5. Lyon: IARC Scientific Publications No. 88, IARC; 1987.  |
| 7. | Parkin DM, Muir CS, Whelan SL, Gao Y-T, Ferlay J, Powell J. Cancer Incidence in Five Continents, Vol. 6. Lyon: IARC Scientific Publications No. 120, IARC; 1992.  |
| 8. | Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. Cancer Incidence in Five Continents, Vol. 7. Lyon: IARC Scientific Publications No. 143, IARC; 1997.  |
| 9. | Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents, Vol. 8; Lyon: IARC Scientific Publications No. 155, IARC; 2002.  |
| 10. | Waterhouse J, Muir CS, Correa P, Powell J. Cancer Incidence in Five Continents, Vol. 3. Lyon: IARC Scientific Publications No. 15, IARC; 1976.  |
| 11. | Waterhouse J, Muir CS, Shanmugaratnam K, Powell J. Cancer Incidence in Five Continents, Vol. 4. Lyon: IARC Scientific Publications No. 42, IARC; 1982.  |
| 12. | Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer 2010;127:2918-27.  [PUBMED] [FULLTEXT] |
| 13. | UN UN. Department of Economic and Social Affairs, Population Division, 2009. World Population Prospects: The 2008 Revision, Highlights, Working Paper No. ESA/P/WP.210. New York: 2008.  |
| 14. | Segi M. Cancer Mortality for Selected Sites in 24 Countries (1950-57). Department of Public Health. Sendai, Japan: Tohoku University of Medicine; 1960.  |
| 15. | Doll R, Payne P, Waterhouse JA. Cancer Incidence in Five Continents. Vol. 1. Geneva: The international union against cancer; 1966.  |
| 16. | Akaza H. Aim of the working group on the Asian perspectives on cancer control: Asian perspective on prostate cancer prevention. Jpn J Clin Oncol 2010;40 Suppl 1:i2-6.  [PUBMED] |
| 17. | Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res 2009;53:171-84.  [PUBMED] |
| 18. | Pu YS, Chiang HS, Lin CC, Huang CY, Huang KH, Chen J. Changing trends of prostate cancer in Asia. Aging Male 2004;7:120-32.  [PUBMED] |
| 19. | Zhang L, Yang BX, Zhang HT, Wang JG, Wang HL, Zhao XJ. Prostate cancer: an emerging threat to the health of aging men in Asia. Asian J Androl 2011;13:574-8.  [PUBMED] [FULLTEXT] |
| 20. | Crawford ED. Epidemiology of prostate cancer. Urology 2003;62(6 Suppl 1):3-12.  |
| 21. | Delongchamps NB, Singh A, Haas GP. The role of prevalence in the diagnosis of prostate cancer. Cancer Control 2006;13:158-68.  [PUBMED] [FULLTEXT] |
| 22. | Namiki M, Akaza H, Lee SE, Song JM, Umbas R, Zhou L, et al. Prostate Cancer Working Group report. Jpn J Clin Oncol 2010;40 Suppl 1:i70-5.  [PUBMED] [FULLTEXT] |
| 23. | Zhang L, Wu S, Guo LR, Zhao XJ. Diagnostic strategies and the incidence of prostate cancer: reasons for the low reported incidence of prostate cancer in China. Asian J Androl 2009;11:9-13.  [PUBMED] [FULLTEXT] |
| 24. | The World Factbook 2009. Washington, DC: Central Intelligence Agency; 2009. Available from: http://www.cia.gov/library/publications/the-world-factbook/index.html. [Last accessed on 2009].  |
| 25. | Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, et al. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci 2010;25:1113-21.  [PUBMED] [FULLTEXT] |
| 26. | Fukagai T, Shimada M, Yoshida H, Namiki T, Carlile RG. Clinical-pathological comparison of clinical prostate cancer between Japanese Americans in Hawaii and Japanese living in Japan. Int J Androl 2000;23 Suppl 2:43-4.  [PUBMED] [FULLTEXT] |
| 27. | Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol 2008;15:3866-71.  |
| 28. | Etzioni R, Legler JM, Feuer EJ, Merrill RM, Cronin KA, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer–part III: Quantifying the link between population prostate-specific antigen testing and recent declines in prostate cancer mortality. J Natl Cancer Inst 1999;91:1033-9.  [PUBMED] [FULLTEXT] |
| 29. | Feuer EJ, Mariotto A, Merrill R. Modeling the impact of the decline in distant stage disease on prostate carcinoma mortality rates. Cancer 2002;95:870-80.  [PUBMED] [FULLTEXT] |
| 30. | Neppl-Huber C, Zappa M, Coebergh JW, Rapiti E, Rachtan J, Holleczek B, et al. Changes in incidence, survival and mortality of prostate cancer in Europe and the United States in the PSA era: additional diagnoses and avoided deaths. Ann Oncol 2011. [In Press].  |
| 31. | Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. JAMA 1995;273:548-52.  [PUBMED] |
| 32. | Peyromaure EM, Mao K, Sun Y, Xia S, Jiang N, Zhang S, et al. A comparative study of prostate cancer detection and management in China and in France. Can J Urol 2009;16:4472-7.  [PUBMED] [FULLTEXT] |
| 33. | Peyromaure M, Debré B, Mao K, Zhang G, Wang Y, Sun Z, et al. Management of prostate cancer in China: a multicenter report of 6 institutions. J Urol 2005;174:1794-7.  |
| 34. | Ogawa K, Nakamura K, Sasaki T, Onishi H, Koizumi M, Araya M, et al. Radical external beam radiotherapy for clinically localized prostate cancer in Japan: changing trends in the patterns of care process survey. Int J Radiat Oncol Biol Phys 2011;81:1310-8.  [PUBMED] [FULLTEXT] |
| 35. | Na YQ. Are we ready for prostate cancer? Chin Med J (Engl) 2008;121:291.  [PUBMED] [FULLTEXT] |
| 36. | Ahmad OB, Bosci-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age Standardization of Rates: a new WHO Standard. In GPE Discussion Paper Series: No. 31. Geneva: World Health Organization; 2000.  |
| 37. | Curado. M. P., Edwards, B., Shin. H.R., Storm. H., Ferlay. J., Heanue. M. and Boyle. P., eds. Cancer Incidence in Five Continents: Vol. 9. IARC Scientific Publications No. 160. Lyon, France: International Agency for Research on Cancer; 2007.  |
Authors
Dr. Jennifer Cullen, PhD, MPH Assistant Professor in Department of Surgery, Uniformed Services University of the Health Sciences (USUHS); Bethesda, MD Director of Epidemiologic Research at the Center for Prostate Disease Research; a program of USUHS; Rockville, MD, East Jefferson Street Rockville, MD
MS. Sally Elsamanoudi, MPH 301-319-2900 Center for Prostate Disease Research, Department of Defense; Rockville, MD Henry M. Jackson Foundation for the Advancement of Military Medicine; Bethesda, MD Walter Reed National Military Medical Center; Bethesda, MD
Dr. David G McLeod, Endowed Clinical Chair at the Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD Director, Center for Prostate Disease Research, Rockville, MD Chief of Urologic Oncology, Walter Reed National Military Medical Center (WRNMMC), Bethesda, MD
Dr. Stephen A Brassell, Center for Prostate Disease Research, Department of Defense; Rockville, MD Uniformed Services University of the Health Sciences; Bethesda, MD Walter Reed National Military Medical Center; Bethesda, MD
Dr. Yongmei Chen, Center for Prostate Disease Research, Department of Defense; Rockville, MD Henry M. Jackson Foundation for the Advancement of Military Medicine; Bethesda, MD
Dr. Monica Colombo, Walter Reed National Military Medical Center; Bethesda, MD
Ms. Amita Srivastava , Center for Prostate Disease Research, Department of Defense; Rockville, MD
Figures
Tables
