Mrinal V Shete1, Revati S Deshmukh1, Tejas Kulkarni2, Anagha V Shete2, Prasad Karande1, Pratik Hande3
1 Department of Oral and Maxillofacial Pathology, Dr. D. Y. Patil Dental School, Pune, Maharashtra, India
2 Department of Oral Medicine and Radiology, Dr. D. Y. Patil Dental School, Pune, Maharashtra, India
3 Department of Oral Surgery, Dr. D. Y. Patil Dental School, Pune, Maharashtra, India
| Date of Submission | 08-Feb-2020 |
| Date of Decision | 25-Feb-2020 |
| Date of Acceptance | 29-Feb-2020 |
| Date of Web Publication | 30-Mar-2020 |
Correspondence Address:
Dr. Mrinal V Shete
Department of Oral and Maxillofacial Pathology and Microbiology, D. Y Patil Dental School, D Y Patil Knowledge City, Charoli Budruk, Lohegaon, Pune – 412 105, Maharashtra
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_3_20
Abstract
BACKGROUND: Cancer invasion is a critical step for tumor growth and its progression. The focus on epithelial changes is shifting to increasing recognition that the microenvironment makes significant contributions to tumor progression. Stromal myofibroblasts play an important role in tumor invasion and metastasis due to its ability to modify the extracellular matrix. Based on this literary evidence, we carried out an immunohistochemical study to observe the expression of myofibroblasts in oral epithelial dysplasia and oral squamous cell carcinoma (OSCC).
AIM: The aim of the study was to evaluate, compare, and correlate the presence of myofibroblasts in normal oral mucosa, oral epithelial dysplasia, and OSCC and to observe different patterns of myofibroblast arrangement using alpha-smooth muscle actin (α-SMA) as a marker, Thus assisting in early diagnosis, treatment, and prognosis of oral carcinomas.
MATERIALS AND METHODS: Thirty-six cases including 12 cases of OSCC, 12 cases of epithelial dysplasia, and 12 cases of normal oral mucosa were stained with hematoxylin and eosin to confirm the diagnosis and immunohistochemically using α-SMA antibody. The slides were evaluated for positivity and intensity of staining.
STATISTICAL ANALYSIS: The result was subjected to statistical analysis using Fisher’s exact test.
RESULTS: α-SMA expression in the stroma of squamous cell carcinoma was greater than its expression in epithelial dysplasia and normal oral mucosa.
Keywords: Alpha-smooth muscle actin, myofibroblasts, oral squamous cell carcinoma
| How to cite this article: Shete MV, Deshmukh RS, Kulkarni T, Shete AV, Karande P, Hande P. Myofibroblasts as important diagnostic and prognostic indicators of oral squamous cell carcinoma: An immunohistochemical study in normal oral mucosa, epithelial dysplasia, and oral squamous cell carcinoma. J Carcinog 2020;19:1 |
| How to cite this URL: Shete MV, Deshmukh RS, Kulkarni T, Shete AV, Karande P, Hande P. Myofibroblasts as important diagnostic and prognostic indicators of oral squamous cell carcinoma: An immunohistochemical study in normal oral mucosa, epithelial dysplasia, and oral squamous cell carcinoma. J Carcinog [serial online] 2020 [cited 2021 Oct 15];19:1. Available from: https://carcinogenesis.com/text.asp?2020/19/1/1/281627 |
Introduction
Oral squamous cell carcinoma (OSCC) is the most frequent type of oral malignancy globally and is associated with a high mortality rate.[1] The progression of carcinomas has conventionally been attributed to a stepwise accumulation of genetic changes within the target epithelium. Such molecular progression has been demonstrated in the oral mucosa where it is initially reflected in the appearance of precursor lesions with epithelial hyperplasia and dysplasia followed later by the development of frank carcinoma, changes paralleled by increase in genetic alterations in the epithelium.[2] However, the focus on solely epithelial changes has begun to change, and a recent paradigm shift leads to increasing recognition that the microenvironment makes significant contributions to tumor progression.[2]
Concurrent with the conversion of nondiseased epithelial tissue to precancerous epithelium to carcinoma, the stroma also changes from normal to “primed” to “activated or tumor associated.” Remodeling of the extracellular matrix (ECM) or “stromagenesis” is initiated by tumor cells, while stromal cells are responsible for the organization of this process. Fibroblasts are considered as one of the most important mesenchymal cells involved in tumor progression.[1] Myofibroblasts are a unique group of cells phenotypically intermediate between smooth muscle cells and fibroblast.[3] In addition to their normal role in tissue homeostasis and repair, altered number and function of myofibroblasts have been implicated in diseases with increased ECM deposition and resultant fibrosis,[4] and now, researchers have started understanding their role in cancers. They modulate the tumor stroma through secretion of a myriad of factors such as chemokines, growth factors, and matrix-degrading enzymes like MMPs.[2] MF are prominent feature of tumor stroma of many but not all OSCCs.[4]
Hence, this study aims to evaluate and compare the presence of myofibroblasts in normal oral mucosa, epithelial dysplasia, and OSCC, considering the vital role of these cells in the progression and aggressive behavior of the tumor.
Materials and Methods
The present study was carried out on 36 archival tissue blocks which included 12 cases of OSCC, 12 cases of epithelial dysplasia, and 12 cases of normal oral mucosa. Four to five serial sections of 5 μ thickness were taken from each block using a soft-tissue microtome (Leica RM 2165, Germany). These consecutive sections of each case were stained employing hematoxylin and eosin (H and E) and using alpha-smooth muscle actin (α-SMA) to demonstrate its expression in the OSCC, epithelial dysplasia, and normal oral mucosa.
The H and E- and α-SMA-stained sections of each case were then viewed under a compound microscope. All the images were captured using a digital camera (Sony Cyber Shot, DSC-W570, Japan) with ×10 and ×40 apochromatic objectives.
To evaluate the accuracy of the study, positive control of ductal carcinoma of the breast (positive α-SMA) was used. Staining of endothelial cells of the blood vessels with α-SMA was used as an internal positive control. Cases of normal oral mucosa with α-SMA served as a control group. The cytoplasm of those α-SMA-stained myofibroblasts in the squamous cell carcinoma (SCC) and in dysplasia and normal oral mucosa located under mucosa was counted in 100 cells at 40-time magnification, and the calculated average number was considered as the percentage of stained cells. The α-SMA-stained endothelial cells of blood vessels were not included in the calculation.
The results were scored as follows: (Kellermann et al., 2007)
- Score 1 (−) (negative): If myofibroblasts were not stained with α-SMA, or if less than 1% of myofibroblasts were stained with α-SMA
- Score 2 (+) (scanty): If more than 1% and <50% of myofibroblasts were stained with α-SMA
- Score 3 (++) (abundant): If more than 50% of myofibroblasts were stained with α-SMA.
Considering the distribution pattern of myofibroblasts, the arrangement of positive-stained cells was classified into three groups
- Focal: If myofibroblasts had a focal arrangement or had no special arrangement in different areas of connective tissue and stroma
- Network: Myofibroblasts with vesicular nucleus and abundant cytoplasm arranged in multiple rows with interwoven network of cytoplasmic extensions forming a network in the stroma of the connective tissue
- Spindle: Myofibroblasts arranged in one to three rows in a regular order in the periphery of the neoplastic islands or in the connective tissues with distinctive cell margins around myofibroblasts and malignant tissue.[5]
Results
Using these scoring criteria, 12 cases of normal oral mucosa, 12 cases of epithelial dysplasia, and 12 cases of OSCC consisting of a total of 36 cases were stained with H and E and α-SMA in our study and the results were recorded.
[Table 1] and [Figure 1] show grade of the lesion versus staining intensity. Of 12 cases of OSCC, eight showed +2 staining [Figure 2]a and b] and four showed +1 staining. Of 12 cases of oral epithelial dysplasia, five showed +1 staining [Figure 3]a and b] and the remaining seven showed 0 staining intensity [Figure 4]a and b]. All the cases in the control group, i.e., in normal oral mucosa showed 0 staining intensity [Figure 5]a and b].
| Table 1: Distribution of patients with respect to staining intensity Click here to view |
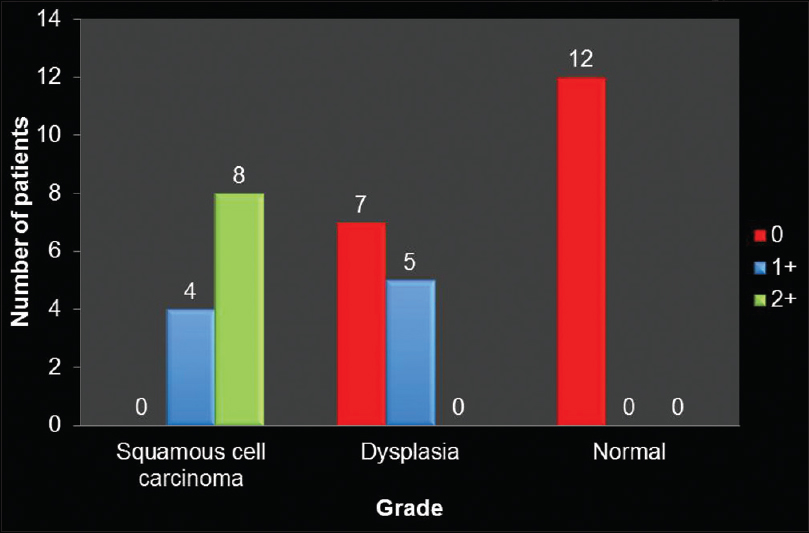 |
Figure 1:Distribution of patients with respect to grade of lesion and staining intensity Click here to view |
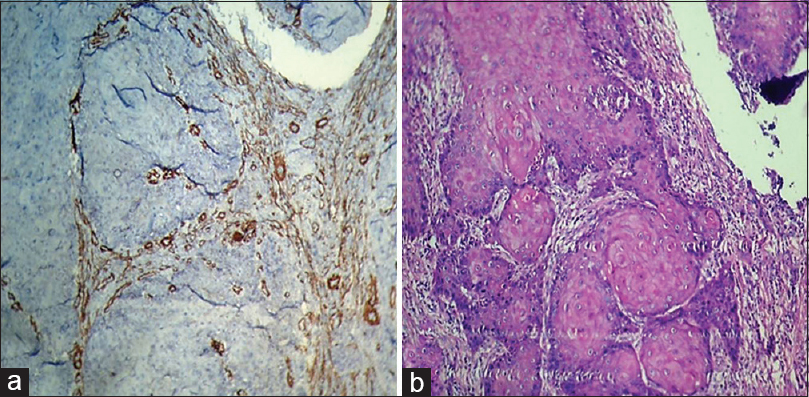 |
Figure 2:(a) Histopathological image shows oral squamous cell carcinoma: Alpha-smooth muscle actin staining intensity +2 (IHC, ×100).,(b) Histopathological image shows oral squamous cell carcinoma (H and E, ×100) Click here to view |
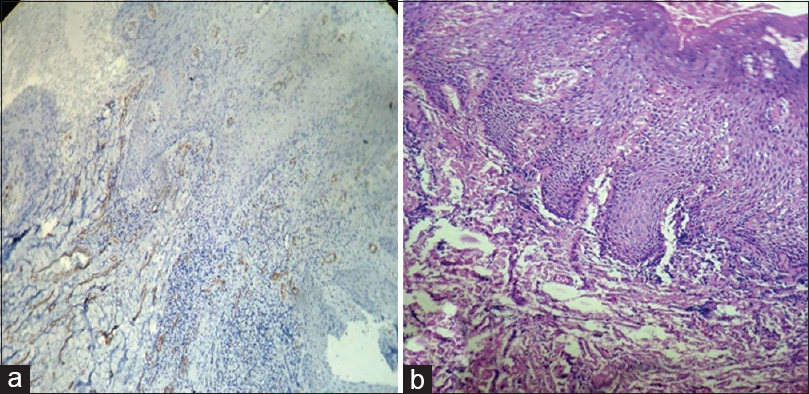 |
Figure 3:(a) Histopathological image shows severe dysplasia: Alpha-smooth muscle actin staining intensity +1 (IHC, ×40). (b) Histopathological image shows severe dysplasia (H and E, ×40) Click here to view |
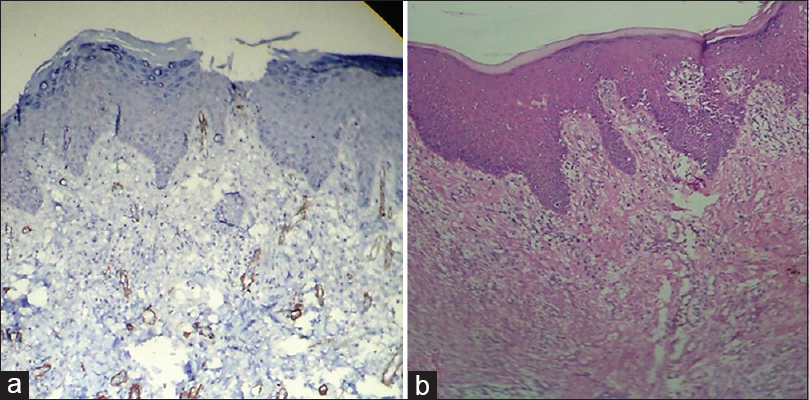 |
Figure 4:(a) Histopathological image shows moderate dysplasia: Alpha-smooth muscle actin staining intensity 0 (IHC, ×40). (b) Histopathological image shows moderate dysplasia (H and E, ×40) Click here to view |
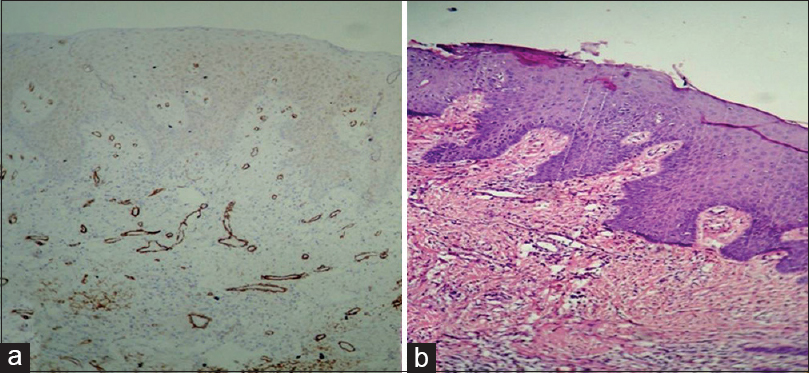 |
Figure 5:(a) Histopathological image shows normal oral mucosa: Alpha-smooth muscle actin staining intensity 0 (IHC, ×40). (b) Histopathological image shows normal oral mucosa (H and E,×40) Click here to view |
Using Fisher’s exact test (P < 0.05), therefore, there is an association between grade of lesion and staining intensity.
[Table 2] and [Figure 6] show the distribution of patterns of myofibroblast arrangement in OSCC cases. Of 12 cases of OSCC, 50% i.e., (six of 12) showed spindle pattern [Figure 7]a and b], 33.3%, i.e., (four of 12) showed a network pattern [Figure 8]a and b], 8.33% i.e., (one out of 12) showed a network + spindle pattern, and 8.33% i.e., (one of 12) showed a focal pattern of arrangement of myofibroblasts [Figure 9]a and b].
| Table 2: Distribution of patterns of myofibroblast arrangement in OSCC Click here to view |
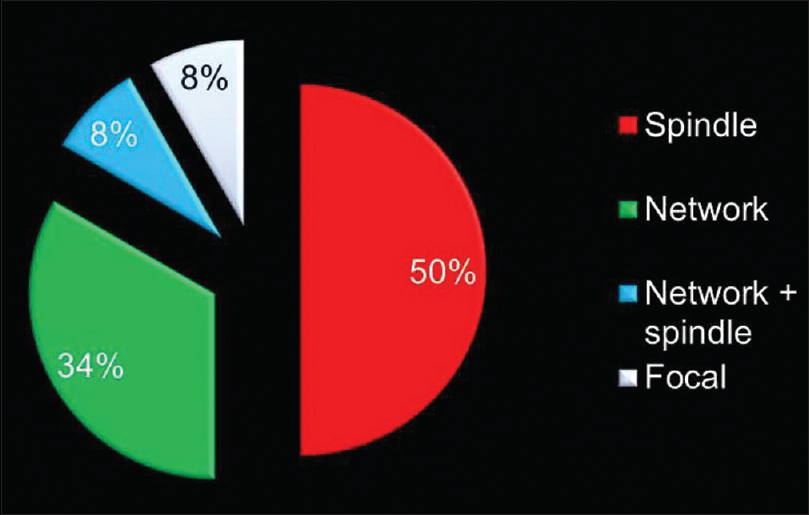 |
Figure 6:Distribution of patterns of myofibroblast arrangement in oral squamous cell carcinoma Click here to view |
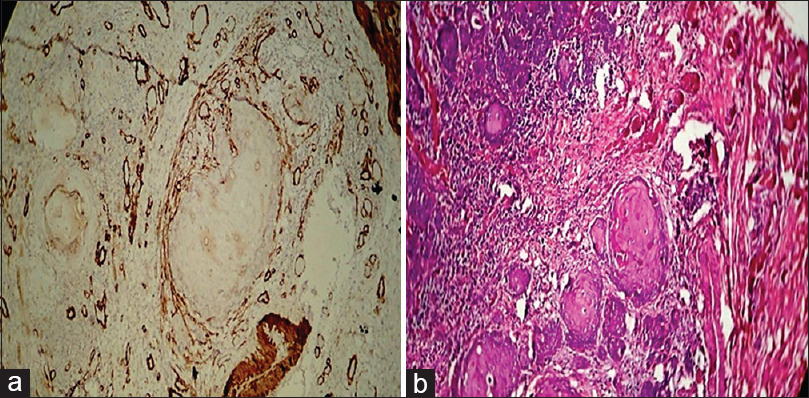 |
Figure 7:(a) Histopathological image shows expression of alpha-smooth muscle actin in oral squamous cell carcinoma (network pattern) (IHC, ×40).(b) Histopathological image shows oral squamous cell carcinoma (H and E, ×40) Click here to view |
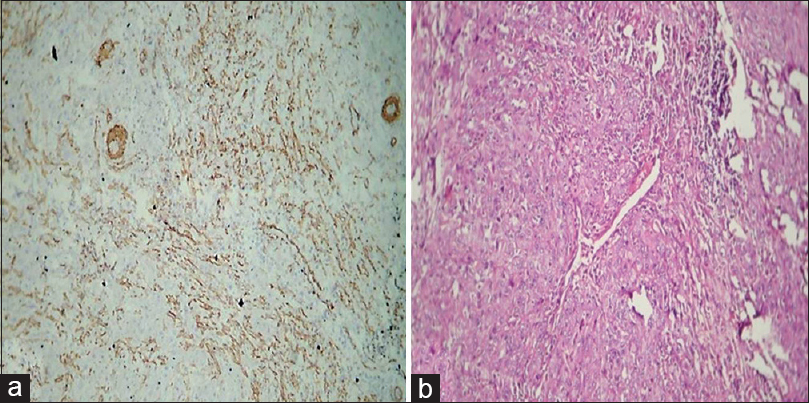 |
Figure 8:(a) Histopathological image shows expression of alpha-smooth muscle actin in oral squamous cell carcinoma (spindle pattern) (IHC, ×40).(b) Histopathological image shows oral squamous cell carcinoma (H and E, ×40) Click here to view |
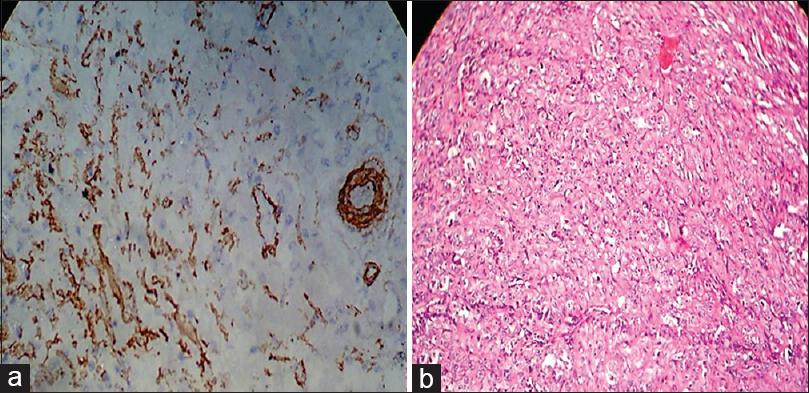 |
Figure 9:(a) Histopathological image shows expression of alpha smooth muscle actin in oral squamous cell carcinoma (focal pattern) (IHC, ×40).(b) Histopathological image shows oral squamous cell carcinoma (H and E, ×40) Click here to view |
Discussion
The following observations were drawn from the present study:
- 100% of cases of OSCC and 33.3% oral epithelial dysplasia cases showed positive staining of α-SMA for myofibroblasts (Fisher’s exact test; P < 0.05, i.e., 0.001 was observed)
- In the OSCC cases, 33% of the cases showed a network pattern of arrangement of myofibroblasts which is proved to be responsible for more invasive behavior of the tumor.
Considering the results we obtained, α-SMA expression in the stroma of squamous epithelial carcinoma was greater than its expression in epithelial dysplasia and normal oral mucosa, which was in accordance with α-SMA positive myofibroblast’s role in invasive behavior of OSCC.
According to the results obtained in this study, the number of myofibroblasts was significantly higher in OSCC compared to dysplastic epithelium and normal mucosa which was devoid of myofibroblasts. These findings were in agreement with those reported by Zidar et al. in 2002[6] who also found a lack of myofibroblasts in normal and dysplastic laryngeal epithelium.
The results of this study showed that there seems to be a relationship between cell arrangement pattern and tumor invasive behavior. Scattered focal arrangement was observed in precancerous lesions, while network and spindle ones were observed in neoplastic lesions. It can be said that because of the higher number of myofibroblasts in network arrangements, they show more severe invasive behavior in comparison to spindle arrangement.[7] Till date, just a few studies are done on the design and arrangement of cells and their role in the invasive behavior of tumors. It seems an area of interest which needs hard work.
In our study, the presence of myofibroblasts did not show a significant difference between the three histologic grades. This was in agreement with the results obtained by Kellermann et al.[7] who were also unable to find a correlation between SCC differentiation and the observation of myofibroblasts. These findings may suggest that the transdifferentiation of myofibroblasts is induced somewhere in the invasive stage of SCC, and further loss of tumoral differentiation (increased grade) would not affect the number of these cells. Statistical evaluation was not performed for the different grades of intraepithelial dysplasias due to the small number of cases.
Considering the design and cell distribution in our study, in dysplasia cases, (33.3%) 4 of 12 cases, in which myofibroblasts staining was positive, showed focal arrangements. Most myofibroblasts were separately and focally arranged and some others had scattered and scanty arrangement around the blood vessels.
Of 12 cases of OSCC [Table 2] and [Figure 6], network arrangement occurred in 33.33%, i.e., 4 cases [Figure 7], spindle arrangement occurred in 50%, i.e., 6 cases [Figure 8], and network + spindle in 8.33%, i.e., 1 case, and focal in 8.33%, i.e., 1 case [Figure 9].
The results of our study were closely similar to other studies of myofibroblasts using α-SMA as a marker. Our results for the staining intensity of myofibroblasts as well as their different distribution patterns were in accordance with the published literature, thus emphasizing the role of myofibroblasts in OSCCs.
In the network configuration, myofibroblasts were arranged in several abundant layers around the neoplastic islands. Increased number of myofibroblasts and their epithelial arrangement was such that in some areas, they were interwoven with neoplastic islands forming a network appearance. In spindle arrangement, the myofibroblasts were arranged in rows with fewer numbers around the neoplastic islands. Considering myofibroblast’s stromal presence and distribution in SCC lesions, it can be said that higher the number of myofibroblasts, the more invasive the tumor behavior was. This is due to the fact that secreted matrix metalloproteinase from myofibroblasts plays a role in tumor invasiveness and its weak prognosis. Matrix metalloproteinase has a role in the destruction of ECM, tumor formation, migration, invasion, metastasis, and angiogenesis and induction of apoptotic clones.
Over the past decade, studies have shown that the microenvironment or stroma of neoplastic tissues plays an active role in tumor progression. Transdifferentiation of fibroblasts to myofibroblasts is a crucial and early event in tumorigenesis, which is mediated by growth factors and cytokines expressed by tumor cells. Myofibroblasts secrete numerous growth factors and inflammatory mediators that stimulate epithelial cell proliferation. Therefore, these cellular elements play an important role in tumoral invasion and use a combination of different factors in the course of neoplastic growth and development.
Hence, considering the vital role of these cells in the progression and aggressive behavior of the OSCCs, this study aims to evaluate and compare the presence of myofibroblasts in normal oral mucosa, epithelial dysplasia, and OSCC, using α-SMA as a marker. To accept or reject the formulated hypothesis,a retrospective study was carried out and the study sample comprised 36 cases – 12 cases of normal oral mucosa, 12 cases of oral epithelial dysplasia, and 12 cases of OSCC.
Various studies reported the presence of stromal myofibroblasts in invasive breast, throat, and larynx cancers (Barth et al., 2004; Yazhou et al., 2004; and Cimpean et al., 2005).
To identify stromal myofibroblasts, Etemad-Moghadam et al. in 2009[1] used α-SMA, vimentin, and desmin markers on 40 samples of SCC, 15 cases of dysplasia, and 15 samples of normal oral epithelium. Stained cells with all three markers were seen in oral cancer, but negative staining presented in dysplasia and normal epithelium. They concluded that the presence of myofibroblasts in the stroma of oral cancer is an expression of their key role in carcinogenesis process.[1]
Cîmpean et al. in 2005 used immunohistochemical (IHC) methods to study the importance of α-SMA and CD34 markers in benign and malignant breast tumors in 112 women patients with a mass in their breasts and reported that the expression of α-SMA was negative in normal breast tissues, but CD34 was positive and concluded that the mentioned markers can be useful in distinction of benign and malignant breast tumors in some severe cases.[8]
Neoplastic changes that happen in the epithelium are followed by some changes in the stroma that are caused by factors such as platelet-derived growth factor and transforming growth factor-β1 from stromal surrounding tumor cells which, in turn, promote the differentiation of fibroblasts into myofibroblasts.[9]
Adegboyega et al. in 2002[10] used α-SMA and vimentin IHC staining on myofibroblasts, for normal colon mucosa, hyperplastic polyps, and colorectal adenomatous in their research. α-SMA-negative fibroblasts and vimentin-positive ones were observed in the colon mucosa, whereas α-SMA- and vimentin-positive fibroblasts were observed in hyperplastic and neoplastic polyps. They concluded that in neoplastic cases, intercellular fibroblasts differentiate into myofibroblasts in the stroma of SCC. They also studied its relationship with the tumor stage and reported that there was a relationship between the expression of α-SMA and tumor stage.
The presence of myofibroblasts in dysplastic and carcinomatous oral epithelium has not been extensively investigated. In 2008, Kellerman et al.[7] studied the presence of myofibroblasts using IHC staining with α-SMA protein in 83 cases of tongue SCC and 34 samples as the control group (8 cases of normal oral mucosa and 16 cases of dysplasia) and reported that the stroma of the oral mucosa and epithelial dysplasia did not occur in any α-SMA cells except for the endothelial cells of blood vessels. However, many myofibroblasts were observed in 60% of the cases of the OSCC. Kellerman’s study results are, in a form, in accordance with the results of our study.
However, in our study, α-SMA-positive myofibroblasts were seen in 100% (12 out of 12) of OSCC and 33.3% (four out of 12) of epithelial dysplasia. Regarding normal oral mucosa, positive staining was seen only in the blood vessels [Table 1] and [Figure 1]. Using Fisher’s exact test (P < 0.05), i.e., <0.001, therefore, there is an association between grade of lesion and staining intensity [Table 1] and [Figure 1], which implies that the nature of the lesion, i.e., from epithelial dysplasia to frank carcinomatous changes, is directly proportional to the staining intensity of the myofibroblasts.
Hence, it is noteworthy that the increase in myofibroblasts found in SCCs may be due to an inducing effect of the carcinomatous component. Epithelial–stromal interactions, different growth factors released by malignant epithelial cells, or numerous other processes may be responsible for the appearance of myofibroblasts.
Shimasaki et al. in 2006[11] confirmed the role of the distribution and arrangement of myofibroblasts in bladder carcinoma and tumor invasive behavior. Fasicular and reticular arrangements were seen in invasive and noninvasive bladder carcinoma, respectively. They concluded that the myofibroblasts distribution pattern can give some information on carcinoma invasion characteristics. A relationship between myofibroblast distribution pattern and invasive tumor characteristics has also been reported by Tuxhorn et al. in 2002.[12]
However, other studies only mentioned an increase in number (percentage) of myofibroblasts in tumor invasion and did not study their cellular distribution pattern.[1],[13]
Using IHC methods, Vered et al. in 2009[14] studied the design and distribution pattern of myofibroblasts. Scanty arrangement was observed in hyperplasia and dysplasia. However, network arrangement and spindle arrangement of SCC were observed in 23% and 77% of cases, respectively. They too confirmed the role of network arrangement in invasive tumor behavior and weak prognosis of oral cancer.
Conclusion
- This study demonstrated that the α-SMA expression in the stroma of squamous epithelial carcinoma was greater than its expression in epithelial dysplasia and normal oral mucosa which is in accordance with α-SMA positive myofibroblast’s role in the invasion of OSCC. In the OSCC cases, 33% of the cases showed network pattern of arrangement of myofibroblasts which is proved to be responsible for more invasive behavior of the tumor
- Considering the lack of myofibroblasts in normal and less number in dysplastic oral epithelium and their significantly high number in OSCC, it can be concluded that the genetically altered epithelium (carcinomatous epithelium) may have an inductive effect on the adjacent stroma to produce myofibroblasts
- However, more sophisticated techniques are suggested to further clarify the exact mechanism by which these important cellular elements exert their effects on stromal and epithelial tissue compartments. In the event that our findings are confirmed, with future investigations, therapeutic targeting of myofibroblasts, their byproducts, or factors responsible for transdifferentiation from fibroblasts to myofibroblasts may be beneficial to OSCC as well as oral dysplastic lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. |
Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: Induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol 2008;44:509-17.
 |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. |
