SV Sowmya1, Roopa S Rao1, Kavitha Prasad2
1 Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences, Bengaluru, Karnataka, India
2 Department of Oral and Maxillofacial Surgery, Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences, Bengaluru, Karnataka, India
| Date of Submission | 25-Nov-2019 |
| Date of Decision | 24-Mar-2020 |
| Date of Acceptance | 31-Mar-2020 |
| Date of Web Publication | 18-May-2020 |
Correspondence Address:
S V Sowmya
Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences, Bengaluru – 560 054, Karnataka
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_16_19
Abstract
CONTEXT: Oral cancer metastasis is the leading cause of death globally. The decision-making on the mode of surgical treatment in clinically negative lymph nodes is challenging.
AIM: The aim of this study was to develop a predictive model using clinical and histopathologic parameters that may help in the assessment of the metastatic risk of oral squamous cell carcinoma (OSCC).
SETTINGS AND DESIGN: Clinical data of histopathologically confirmed primary OSCC from 2014 to 2017 were retrieved from the archives. Histopathological parameters for metastasis that were considered for evaluation in the study were tumor buds, cytoplasmic pseudofragments, tumor grade, depth of invasion, invasive tumor front (ITF) pattern, and lymphovascular invasion (LVI).
METHODS: Hematoxylin and eosin and pan-cytokeratin immunostained sections of metastatic and nonmetastatic OSCC were assessed for histopathological features and correlated with clinical parameters.
STATISTICAL ANALYSIS USED: SPSS software (Statistical Package for Social Sciences for Windows, Version 22.0 (2013) (IBM Corp., Armonk, NY, USA)) was used for the statistical analysis. Pearson’s Chi-square test was done to assess the grades of histopathological and clinical parameters between the study groups. Univariate analysis was performed to develop a clinicopathologic predictive model.
RESULTS: The clinicopathologic model signifies that OSCC with clinical Stage IV, high grades of tumor buds and cytoplasmic pseudofragments, Type V ITF pattern, positive LVI, deeply invasive tumors, and poorly differentiated grades of OSCC have a high risk of developing nodal metastasis. These parameters may be used as early predictors for metastasis of OSCC both in incisional and excisional biopsy specimens.
CONCLUSIONS: The proposed predictive model is simple, cost-effective, and user-friendly for the early assessment of nodal metastatic risk in clinically negative lymph nodes.
Keywords: Histopathological variables, lymph node metastasis, predictive model, risk factors, squamous cell carcinoma
| How to cite this article: Sowmya S V, Rao RS, Prasad K. Development of clinico-histopathological predictive model for the assessment of metastatic risk of oral squamous cell carcinoma. J Carcinog 2020;19:2 |
| How to cite this URL: Sowmya S V, Rao RS, Prasad K. Development of clinico-histopathological predictive model for the assessment of metastatic risk of oral squamous cell carcinoma. J Carcinog [serial online] 2020 [cited 2021 Oct 15];19:2. Available from: https://carcinogenesis.com/text.asp?2020/19/1/2/284551 |
Introduction
Cancer risk prediction models have been formulated so far to assess the cost of population prevention strategies, genetic counseling, planning trials, etc.[1] Literature search has revealed the use of a variety of statistical models to predict metastasis and survival with the analysis of various clinicopathological variables and individual biomarkers. However, due to its multifactorial etiology, it is difficult to recognize a single etiological factor for oral cancer metastasis.[2]
Early prediction of metastasis to lymph nodes is a significant oncological factor for the prognosis of oral squamous cell carcinoma (OSCC). At present, various molecular mechanisms have been identified for metastasis, and numerous biomarkers have been investigated for specific mechanisms. It becomes really challenging for a pathologist to predict the metastatic potential of a particular case as it can have serious implications on treatment. The expression of many biomarkers may correlate with the histopathological features, clinical staging, and behavior of the malignancy. The biomarker-based approach may not be affordable to the general public with a need to identify simple and cost-effective modalities for the early prediction of metastatic potential of OSCC.[3] Literature search has revealed that providing effective treatment for OSCC, tumor differentiation, and regional metastasis to the lymph nodes act as reliable predictors. Therefore, research in predicting the metastatic risk of OSCC preoperatively has a significant scope.
Although major progress has been achieved in the surgical procedures and chemotherapy, OSCC continues to have poor survival and prognosis. The challenges in providing treatment amplify with the complex anatomy of the head and neck region and late presentation of the patients to health care.[4] The unaffordable advanced treatment procedures add to the increased mortality rate. The need of the hour is to develop a simple, cost-effective metastatic risk assessment model using only clinical and histopathologic parameters. Therefore, an attempt has been made to develop a model using clinical and histopathologic parameters that may help the clinician in customizing the treatment plan for each case.[2]
Methods
Clinical data were retrieved from the Department of Oral Pathology and Microbiology. Patients with histopathologically confirmed primary OSCC who had undergone surgical resection with neck dissection from 2014 to 2017 were included for the study. Ethical clearance was obtained from the University Ethics Committee for Human Trials, the institutional review board (UECHT/2016-18/PhD_15DSDS095004). The clinical parameters recorded were the age at first diagnosis, gender, site of the primary lesion, tobacco habits, and clinical staging (According to the TNM classification of Union for International Cancer Control).[5] The OSCC cases were further categorized into <45 years and >45 years based on the available literature.
Histopathological parameters for metastasis that were considered for evaluation were tumor buds, cytoplasmic pseudofragments, tumor grade, depth of invasion, invasive tumor front (ITF) pattern, and lymphovascular invasion (LVI). Tumor buds and cytoplasmic pseudofragments were recognized using immunostaining for pan-cytokeratin.
The standard protocol for immunohistochemistry (IHC) was followed. 4 μm sections of 45 selected tissues were deparaffinized at 56°C–60°C for 15 min and transferred to xylene bath. Rehydration with descending grades of ethanol followed by immersion in 0.3% hydrogen peroxide and methanol to abolish endogenous peroxidase activity was done. The sections were heated in a microwave oven for 20 min to facilitate antigen retrieval. Subsequently, primary monoclonal antibodies, anti-pan-cytokeratin (pan-CK (AE1/AE3), rabbit/mouse, 1:50 dilution; Dako, Denmark), diluted in 1% bovine serum albumin and phosphate-buffered saline, was added and incubated overnight at 4°C. IHC staining was done with the Envision system (HRP-based two-step IHC staining method). Breast carcinoma tissues were used as positive controls. Anti-rabbit IgG peroxidase-linked secondary antibodies at 1:100 dilutions were used. The sections were subjected to streptavidin-conjugated Horseradish peroxidase (HRP), visualized using diaminobenzidine tetrahydrochloride with tris buffer, counterstained by Meyer’s hematoxylin, dehydrated in graded alcohols and mounted in DPX.[6],[7]
Single cells or tiny groups of about five or more cells scattered at the ITF were considered as tumor buds. They were graded as low and high based on <5 buds and ≥5 buds, respectively at ×20.[8] Cytoplasmic pseudofragments were identified as non-nucleated, smoothly contoured fragments that were uniformly positively stained for cytokeratin. Based on the number of fragments/field in ×20, they were categorized into low (0–9 fragments) and high grades (≥10 fragments).[9]
Modified Broder’s system was used for assessing the level of tumor differentiation and graded as well, moderate, and poorly differentiated OSCC.[10] The presence of tumor cells in the lymphatic or vascular channels were represented as positive or negative LVI.[11] ITF pattern was graded as Type 1 (broad pushing margin of invasion), Type 2 (finger-like proliferation), Type 3 (larger tumor islands), Type 4 (tumor strands), and Type 5 (tumor satellites).[12] The depth of invasion was measured from the basal layer of the surface epithelium to the deepest point of tumor invasion on the photomicrographs and recorded in millimeters.[13] It has further been classified as less invasive ≤5 mm, moderate invasive 6–10 mm, and deeply invasive ≥10 mm, according to AJCC.[13] Photomicrographs were obtained using a charge-coupled device color video camera-Jenoptik Progres Gryphax Arktur USB 3.0 microscope camera, Jena, Germany attached to the Olympus research microscope (BX53F2, Tokyo) and were saved as 24 bits color 2080 × 1542 bit map image (bmp) file format in a computer. The depth of invasion was measured using Jenoptik Gryphax software V1.1.010.6 (Genoptik Optical Systems GmbH, Jena, Germany).
Recurrent OSCC and insufficient data cases were excluded from the study. The Statistical Package for the Social Sciences for Windows, Version 22.0 (2013) (IBM Corp., Armonk, NY, USA), was used to perform statistical analyses. A Chi-square test was used to compare the clinical and histopathological findings between metastatic and nonmetastatic groups. Univariate analysis was performed to recognize the important predictors from clinical and histopathological parameters to develop a combined clinicopathologic predictive model, wherein the odds ratio (OR) with 95% confidence interval (CI) was calculated for each of the variable. Value of P< 0.05 was considered as statistically significant.
Results
One hundred and seventeen cases of OSCC were screened for all of the following histopathological parameters, tumor buds [Figure 1]a and [Figure 1]b, cytoplasmic pseudofragments [Figure 1]c, tumor grade [Figure 2], LVI [Figure 3]a, depth of invasion [Figure 3]b, and ITF pattern [Figure 4]. Patients with inadequate clinical data such as age, gender, site of the lesion, and staging were excluded from the study. Only 40 cases showed all of the above mentioned histopathologic and clinical features. They were further grouped into 20 cases each of metastatic and nonmetastatic OSCC. Twelve cases (60%) of metastatic OSCC were >45 years at the time of diagnosis, and the majority of them were females (60%). Buccal mucosa (75%) followed by the tongue (15%) and alveolus (10%) were the predominant sites of metastatic OSCC in this study. The study revealed that 85% of the metastatic patients presented to clinicians at stage 4. 60% of metastatic OSCC cases showed smokeless tobacco habit [Table 1].
| Figure 1: Photomicrographs of oral squamous cell carcinoma showing tumor buds in (a) H and E (×40) and (b) immunostaining with Pan CK (×40); (c) cytoplasmic pseudofragments (Pan CK immunohistochemistry; ×200) Click here to view |
| Figure 2: Photomicrographs showing grades of oral squamous cell carcinoma; well differentiated (a); moderately differentiated (b); poorly differentiated (c) (H and E; ×40) Click here to view |
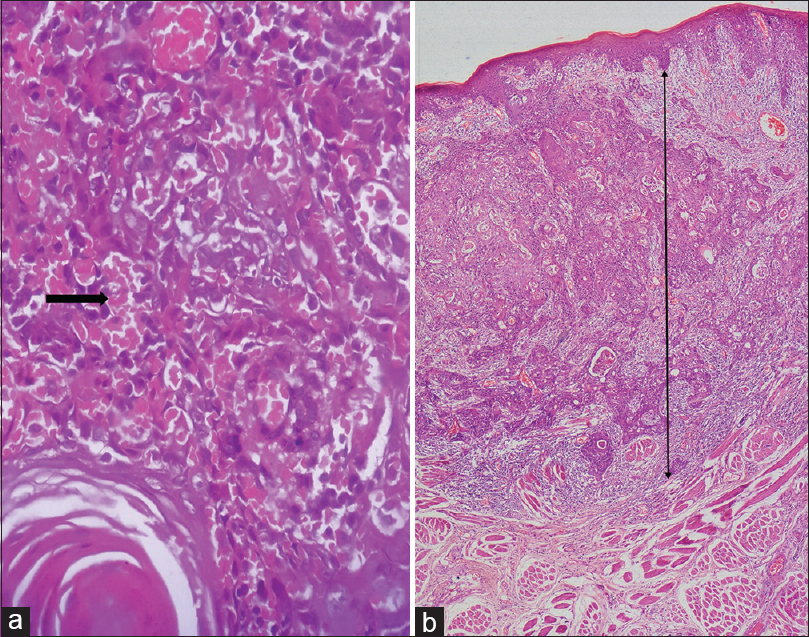 |
Figure 3: Photomicrographs of oral squamous cell carcinoma showing lymphovascular invasion (black arrow) in (a) (H and E; ×400) and measurement of depth of invasion in (b) (H and E; ×40) Click here to view |
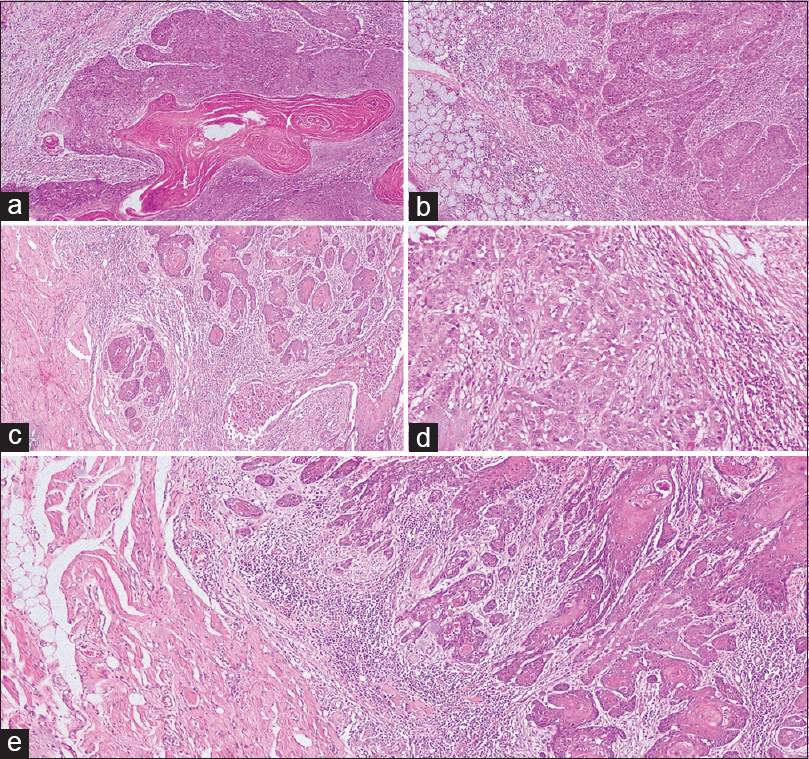 |
Figure 4: Photomicrographs of oral squamous cell carcinoma showing invasive tumor front patterns; (a) Type 1 pattern with broad pushing margins; (b) Type 2 pattern with pushing finger-like margins; (c) Type 3 with invasive islands; (d) Type 4 showing strands of tumor cells; (e) Type 5 with tumor satellites of varying sizes (H and E; ×40) Click here to view |
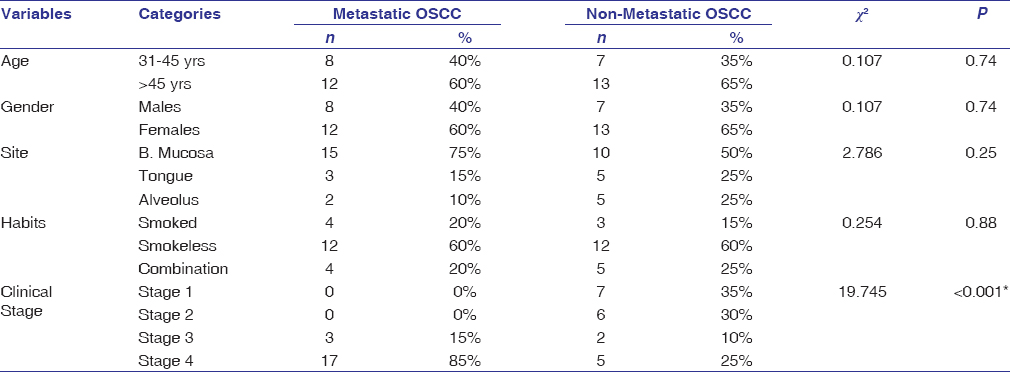 |
Table 1: Univariate relationships between clinical parameters and the presence of lymph node metastasis in OSCC Click here to view |
The study samples were dichotomized age-wise as 31–45 years and >45 years. Patients aged >45 years showed a significant association with the grade of tumor buds (P = 0.003), cytoplasmic pseudofragments (P = 0.02), and tumor grade (P = 0.03) in metastatic OSCC. ITF pattern had a statistically significant association with both the age groups (P = 0.02 and 0.003). The younger age group interestingly revealed significant association (P = 0.03) with LVI between metastatic and non-metastatic groups. Both the age groups had a significant association with depth of invasion on the comparison between metastatic and non-metastatic OSCC groups (P = 0.002 and 0.007) [Table 2].
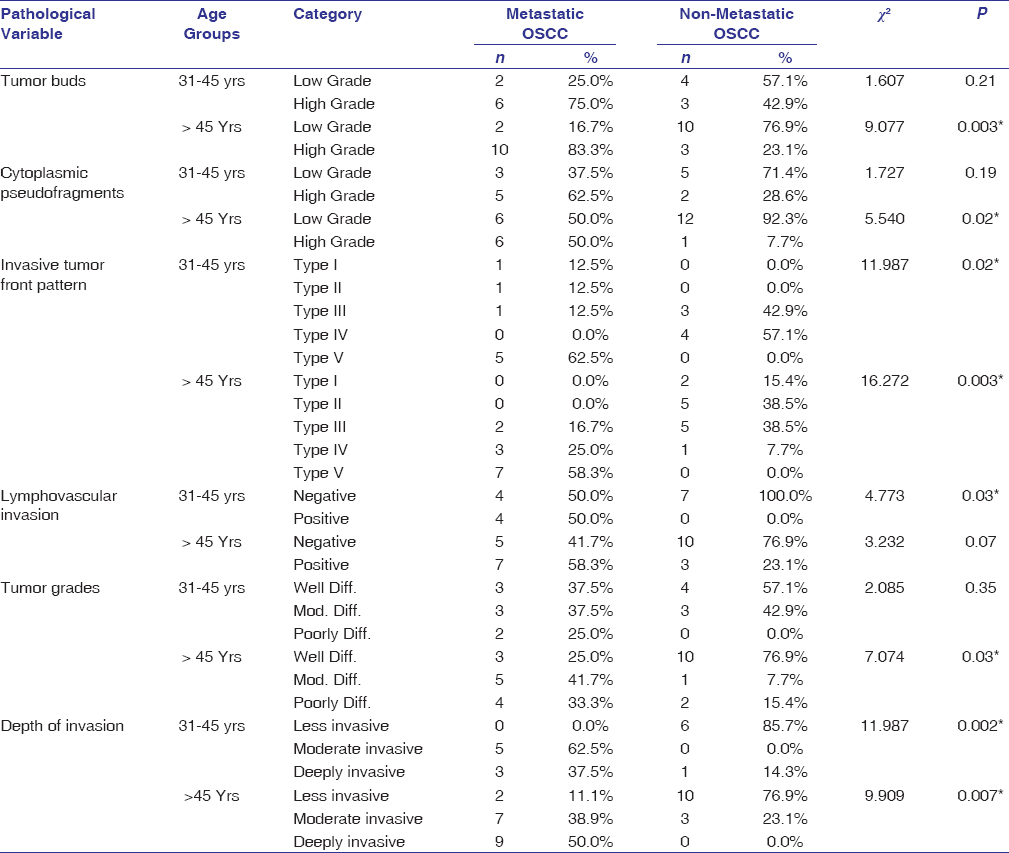 |
Table 2: Age-wise comparison of histopathological variables between metastatic and non-metastatic OSCC Click here to view |
In female gender, there was a significant difference in the number of tumor buds (P = 0.009), grade (P = 0.008), and ITF pattern (P = 0.01), whereas males showed a difference between the study groups with respect to cytoplasmic fragments (P = 0.005) and ITF pattern (P = 0.005). However, there was no significant association of gender observed with LVI between the study groups. Both males and females showed a significant association (P = 0.008 and P = 0.001, respectively) with the depth of invasion on comparison between the study groups [Table 3].
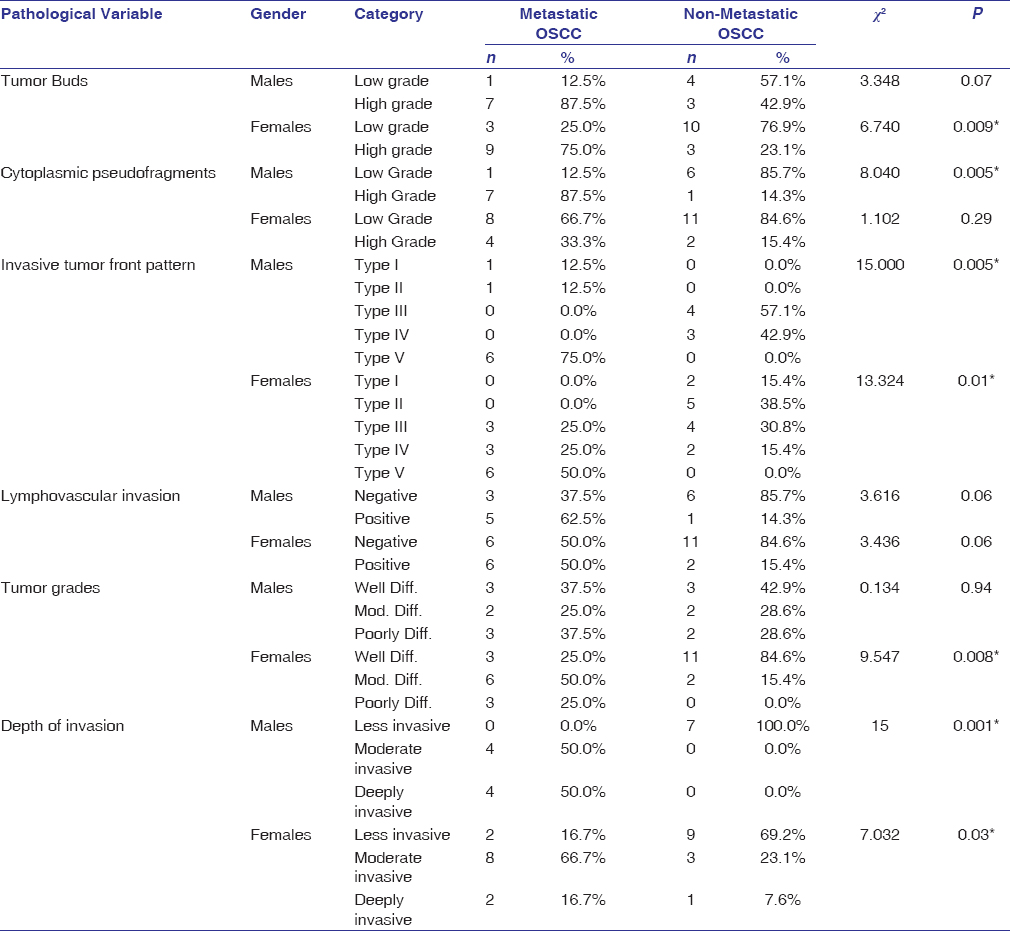 |
Table 3: Gender-wise comparison of histopathological variables between metastatic and non-metastatic OSCC Click here to view |
OSCC patients with smokeless tobacco habit showed a significant difference in the tumor buds (P = 0.01), grade (P = 0.01), and ITF pattern (P = 0.02) between the study groups, whereas cytoplasmic pseudofragments (P = 0.008) was associated with patients having smoking habit. However, combined smoking and smokeless tobacco habit patients showed a difference in tumor buds (0.02) between the study groups. The three habit groups showed a significant association of depth of invasion when compared between metastatic and non-metastatic OSCC groups (P = 0.03, 0.045, and 0.011) [Table 4].
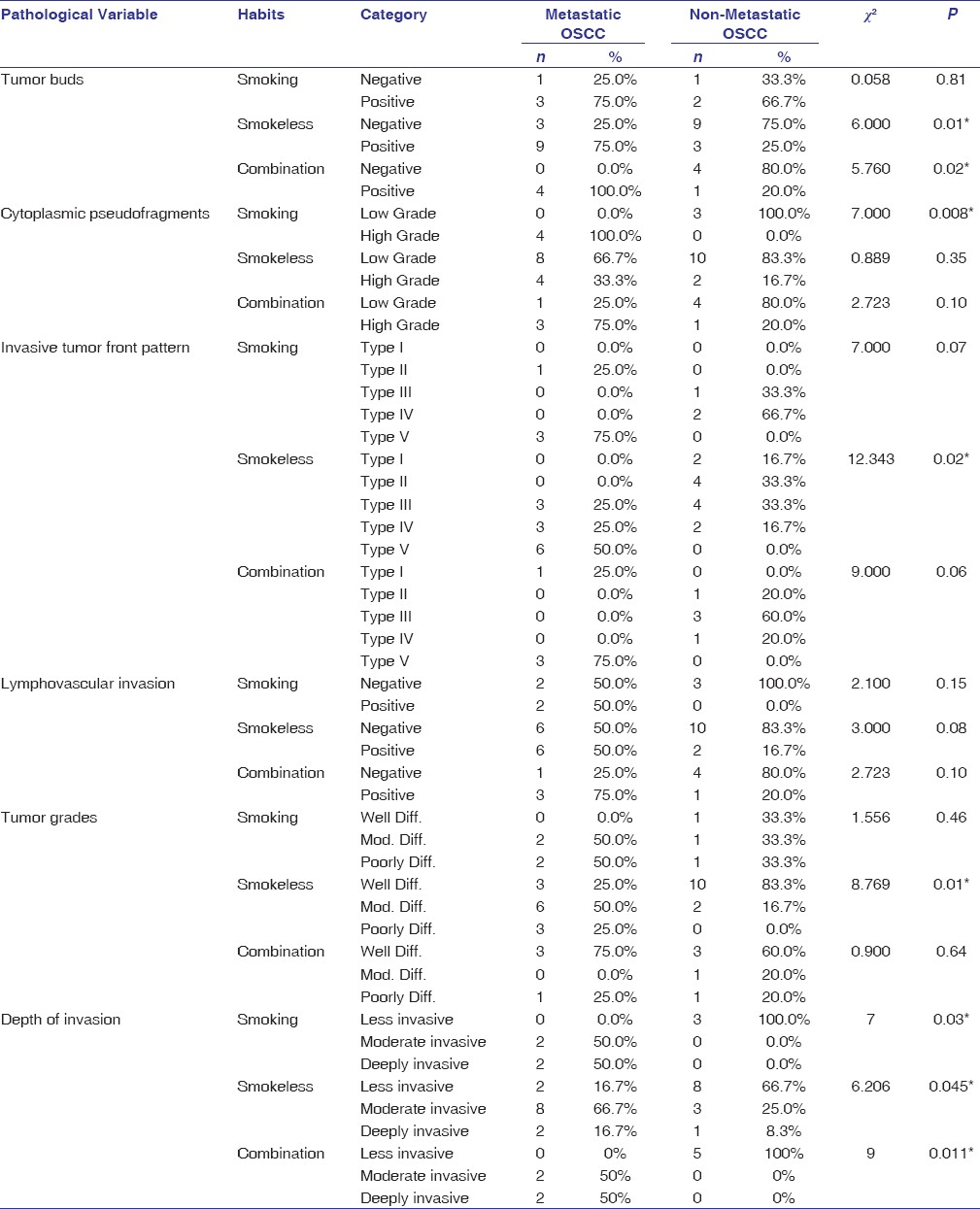 |
Table 4: Comparison of histopathological variables based on the type of habit between metastatic and non-metastatic OSCC Click here to view |
The tissue specimens from buccal mucosa had a significant association with grades of tumor buds (P = 0.01), cytoplasmic pseudofragments (P = 0.005), invasive tumor pattern (P = 0.02), and depth of invasion (0.001) between metastatic and non-metastatic groups. The tongue also showed association with LVI (P = 0.005), ITF pattern (P = 0.02), and depth of invasion (P = 0.018) between the study groups. However, tissue specimens from the alveolus revealed a statistically significant association with tumor grade (P = 0.008) between the groups [Table 5].
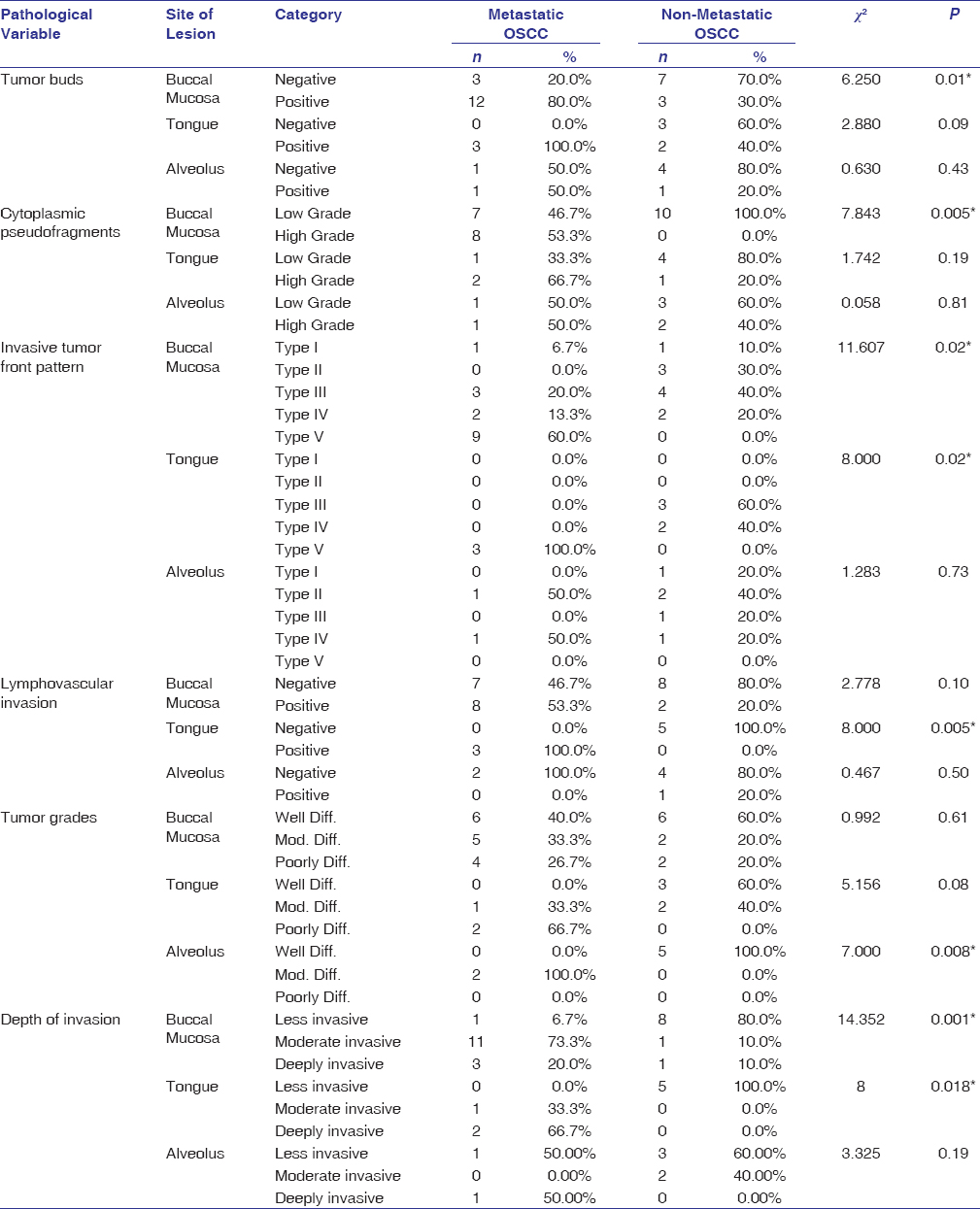 |
Table 5: Comparison of histopathological variables based on the site of lesion between metastatic and nonmetastatic OSCC Click here to view |
Stage IV OSCC specimens showed a significant association with the grade of tumor buds (P = 0.02), ITF pattern (P = 0.03), depth of invasion (P = 0.01), and LVI (P = 0.02) between the study groups [Table 6].
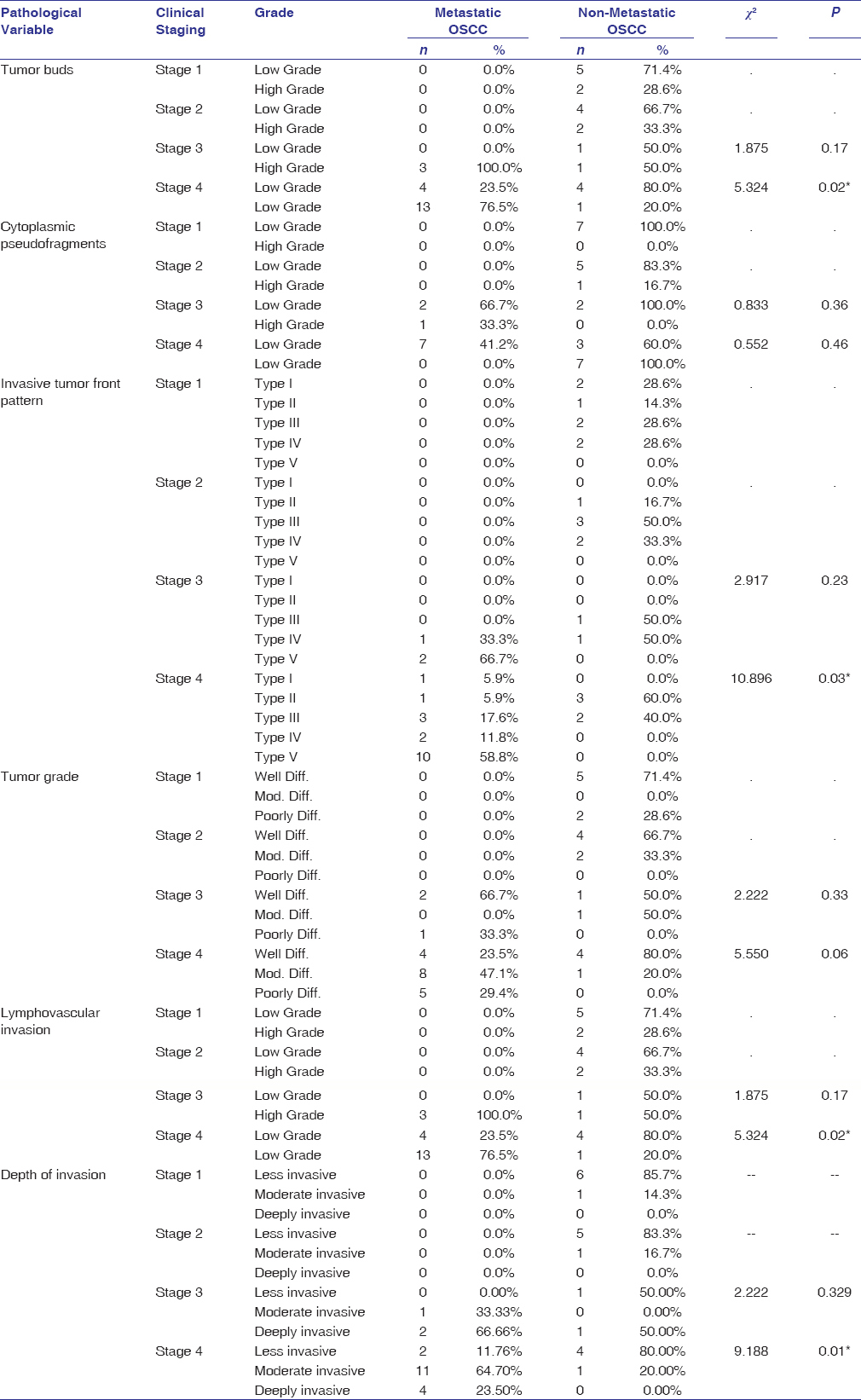 |
Table 6: Comparison of histopathological variables based on the clinical staging between metastatic and nonmetastatic OSCC Click here to view |
Univariate logistic regression found an OR of 1.16 (95% CI: 0.27 ± 4.92) for metastatic patients >45 years relative to those between 31 and 45 years with a P = 0.84. OR for metastasis in males was 0.86 (95% CI: 0.20 ± 3.68) relative to females with a P = 0.74. The OR for metastasis in patients with smokeless and combined tobacco habits relative to smoking was 1.33 (95% CI: 0.24 ± 7.28) and 1.67 (95% CI: 0.23 ± 12.22), respectively. There was an OR of 0.27 (95% CI: 0.04 ± 1.65) and 0.67 (95% CI: 0.08 ± 5.88) for metastasis from the buccal mucosa and tongue relative to alveolar mucosa site with a P = 0.25. OR for metastasis of Stages II, III, and IV were 1.95 (95% CI: 0.84 ± 4.32), 3.67 (95% CI: 1.24 ± 6.71) and 6.28 (95% CI: 3.75 ± 10.92), respectively, relative to Stage I with a significant P< 0.001 [Table 7].
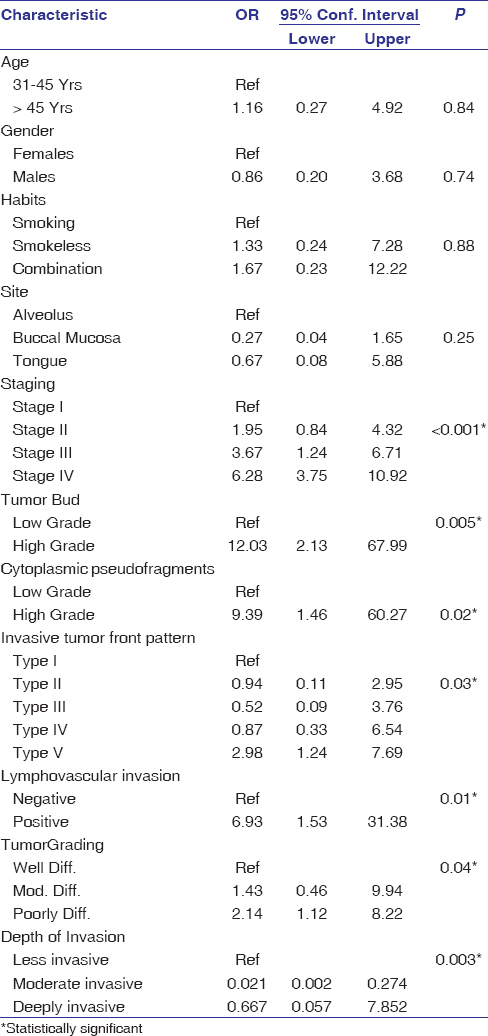 |
Table 7: Univariate regression model for the assessment of metastatic risk of OSCC using the clinical and histopathologic parameters Click here to view |
The OR for metastasis of high-grade tumor buds and cytoplasmic pseudofragments was found to be 12.03 (95% CI: 2.13 ± 67.99) and 9.39, respectively, relative to low grade with significant P = 0.005 and 0.002. Regression analysis also revealed OR for metastasis of Types II, III, IV, and V ITF patterns to be 0.94 (95% CI: 0.11 ± 2.95), 0.52 (95% CI: 0.52 ± 0.09), 0.87 (95% CI: 0.33 ± 6.54), and 2.98 (95% CI: 1.24 ± 7.69), respectively, relative to Type I pattern that was statistically significant (P = 0.03). Metastasis of OSCC for positive LVI cases showed a statistically significant OR of 6.93 (95% CI: 1.53 ± 31.38) relative to negative cases (P = 0.01). OR for metastasis of moderately and poorly differentiated OSCC was 1.43 (95% CI: 0.46 ± 9.94), and 2.14 (95% CI: 1.12 ± 8.22), respectively, relative to well-differentiated tumors (P = 0.04) [Table 7]. Depth of invasion had an OR of 0.021 (95% CI: 0.002 ± 0.274) and 0.667 (95% CI: 0.667 ± 0.057) for moderate and deeply invasive cases relative to the less invasive cases for metastasis of OSCC.
The significant clinical and histopathological variables were considered for developing a combined predictive model. The combined clinicopathologic risk predictive model reveals that OSCC patients with clinical Stage IV, high-grade tumor buds and cytoplasmic pseudofragments, showing Type V ITF pattern, positive LVI, poorly differentiated grade of OSCC, and deeply invasive depth have a higher probability of developing metastasis [Table 8].
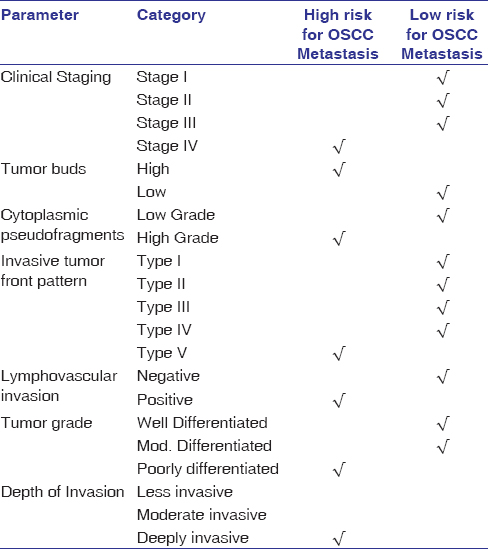 |
Table 8: Combined clinicopathologic metastatic risk predictive model for OSCC Click here to view |
Discussion
Despite advanced treatment strategies, the prognosis and survival rates of OSCC are poor due to regional metastasis and recurrence. The potential for lymph node metastases accounts for 40% and manifests with hidden lymph node involvement in 15% to 34% of the cases.[14] Although elective neck dissection in the clinically negative neck has shown promising results, it is associated with serious complications. It has also been observed that cases presenting clinically with negative nodes have shown positive histopathological lymph nodes proving the concept of occult lymph node metastasis, which has been reported in 15%–60% of cases.[3] This mismatch in the clinical and pathological diagnosis can affect the prognosis of OSCC.[15] The routine treatment modalities varying from neck dissection, elective irradiation, and combination of surgery with radiotherapy can lead to severe functional and cosmetic morbidity.[16],[17]
There are many histopathological, immunohistochemical and genetic variables in the primary tumor that may predict the incidence of lymph node metastasis.[14],[18] Many individual predictive models have been developed in the past that can assess the metastatic risk or survival of OSCC patients.[2] However, there are meager studies that have employed combined clinical and histopathological variables to assess the metastatic risk of OSCC. Hence, there is a need to identify the risk factors involved in metastasis for the development of therapeutic strategies to improve prognosis and survival in OSCC patients.
In the current study, 77.5% of the patients were above the age of 45 years. OSCC is a disease of the elderly and supports the above statement. The association of metastasis with age >45 years well correlated with few histopathological parameters such as tumor buds, cytoplasmic pseudofragments, and grade for OSCC. The presence of significantly high grades of tumor buds, cytoplasmic pseudofragments, and poor differentiation of OSCC was observed in metastatic OSCC, suggesting that they are strong metastatic predictors.[19] Tumor buds and pseudofragments together represent different mechanisms of tumor aggressiveness.[20] The risk of metastasis of moderately and poorly differentiated OSCC is high in the present study and is dependent on the degree of differentiation and the amount of keratinization.[2] The younger patients with positive LVI had a high risk for metastasis that has been documented in previous studies as poor prognostic indicators.[21]
OSCC has been generally documented to be affecting males more than females with an approximate ratio of 1.5:1.[21] However, the present study shows an increased predilection in females (62.5%) and did not differ significantly between metastatic and nonmetastatic groups. This may be attributed to the fact that in the Indian population, the main etiological factor for OSCC is positive tobacco habits, of which the use of betel-quid is prevalent among females. Women who have had hysterectomies and who therefore become menopausal at a younger than average age have a higher tendency to develop OSCC. This suggests that estrogen deficiency may play a role in the pathogenesis of OSCC in some women.[22] Due to the increase in the incidence of oral cancer in females, in our study, we found the higher metastatic potential was associated with high-grade tumor buds, Type V ITF pattern, and poorly differentiated OSCC.
A study by Wu et al., 2019 has reported that smoking and alcohol have a high risk for metastasis.[23] However, Salian et al., 2016 have found quid chewing to be a high-risk etiological factor for OSCC followed by smoking in the Indian scenario.[24] There is no enough literature evidence on the relationship between tobacco habits with respect to histopathological features in metastasis of OSCC. However, the current study showed that smokeless tobacco habit was associated with an increased number of tumor buds, high tumor grade, deeply invasive type of depth of invasion, and Type V ITF pattern.
Acharya et al. 2019 have found 23 OSCC cases out of 40 to be involving buccal mucosa and gingivobuccal sulcus, followed by retromolar trigone (6 cases), tongue (6 cases), lips (3 cases), and palate (2 cases).[25] Chatterjee et al. 2019 evaluated 126 cases of buccal mucosa and tongue for histopathologic variables and found histological grade, the worst pattern of invasion, tumor budding, LVI, and perineural invasion were significantly associated with risk of lymph node metastasis.[19] The present study also showed a significant association of metastatic buccal mucosa SCC cases with high grades of tumor buds, cytoplasmic pseudofragments, deeply invasive depth, and ITF pattern suggesting that these histological variables are high-risk predictors of metastasis.
Siriwardena et al., 2018 have found in their study that patterns of invasion and metastasis were significantly associated with patterns III and IV, revealing higher metastatic rates compared to patterns I and II.[2] The present study showed Stage IV OSCC to be significantly related to the high grade of tumor buds, Type V ITF pattern, deeply invasive depth, and positive LVI.
The current study results revealed a strong association of metastasis in patients with clinical staging IV using a univariate regression model. However, age, gender, habits, and site did not show significant association with metastasis and were therefore, not included in the combined clinicopathologic metastatic risk predictive model. The histopathological variables that showed a strong association with metastasis using the univariate regression model were tumor buds, cytoplasmic pseudofragments, ITF pattern, LVI, depth of invasion, and tumor grading. Hence, they were considered in the clinicopathologic metastatic risk predictive model. The increase in (Depth of invasion) DOI and proliferation of microvasculature suggest proximity of tumor cells to blood and lymph vessels, thereby facilitating the process of metastasis.[26] The initial step toward metastasis is manifested phenotypically in the form of buds actively moving away from the primary tumor.[27] Tumor buds are believed to be dissociated tumor cells invading into the adjacent stroma.[28] Cytoplasmic pseudo-fragments are cytoplasmic processes arising from tumor cells and are markers of dynamic interaction between cancer cells and host tissue.[20] The differentiation of tumor cells at the ITF is at a lower degree with a higher grade of cellular dissociation, providing prognostic information about the tumor’s invasive, aggressive, and metastatic potential.[29]
Recognizing the right predictive model has a direct effect on the type of surgical management. The present model minimizes the need for the use of sophisticated, advanced, and expensive genomic techniques for the identification of metastatic risk of OSCC. Every OSCC case undergoes a thorough clinical examination with an incisional biopsy procedure mandatorily. If the high-risk variables can be identified at this stage of incisional biopsy, the metastatic potential can be predicted at an early stage using routine histopathological techniques. This can minimize the time scheduled for surgery, thus avoiding unnecessary complications due to delay in surgical decision-making, which is dependent on the pathologist’s report. The drawback of this study is the smaller sample size and difficulty in measuring exact DOI in few cases. The strength of the study is that it is easy to use in routine clinical practice and navigates the surgeon to perform appropriate treatment.
Conclusions
The proposed model is simple, cost-effective, and user-friendly for the prediction of nodal metastatic risk in clinically negative lymph nodes. The model is the first of its kind that has made use of combined clinical and histopathological features for the metastatic risk assessment of OSCC. The use of grade of tumor buds in this model makes it a unique one which has not been employed in predictive models proposed so far. The combined clinicopathologic model signifies that OSCC with clinical stage IV, high grades of tumor buds and cytoplasmic pseudofragments, Type V ITF pattern, positive LVI, deeply invasive tumors, and poorly differentiated grades of OSCC have high chances of developing nodal metastasis. These parameters can, therefore, be used as early predictors for metastasis of OSCC both in incisional and excisional biopsy specimens.
This model can be efficiently used in developing countries like India with low socioeconomic status, lacking effective accessibility to health-care services as it does not require advanced molecular techniques and is dependent on the evaluation of simple histopathological and clinical parameters. Further research involving the validation of the predictive model by blinding the samples and the use of larger sample size may be performed.
Acknowledgments
The authors sincerely thank Mr. Chetan Kumar and Mr. Raju, technicians, for performing staining procedures.
Financial support and sponsorship
Self.
Conflicts of interest
There are no conflicts of interest.
References
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. |
Salian V, Dinakar C, Shetty P, Ajila V. Etiological trends in oral squamous cell carcinoma: A retrospective institutional study. Cancer Transl Med 2016;2:33.
 [Full text] |
| 25. |
Acharya S, Kumari N, Srivastava P, Arnold D, Nikhil K. Architectural changes in the regional lymph nodes of oral squamous cell carcinoma. J Oral Maxillofac Pathol 2019;23:305.
 [PUBMED] [Full text] |
| 26. | |
| 27. | |
| 28. | |
| 29. |
Sharma M, Sah P, Sharma SS, Radhakrishnan R. Molecular changes in invasive front of oral cancer. J Oral Maxillofac Pathol 2013;17:240-7.
 [PUBMED] [Full text] |
