Shalinee Rao1, Michael Leonard Anthony1, Nilotpal Chowdhury1, Rajesh Kathrotia2, Mayank Mishra3, Manisha Naithani4, Girish Sindhwani3, Neha Singh1
1 Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
2 Department of Physiology, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
3 Department of Pulmonary Medicine, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
4 Department of Biochemistry, All India Institute of Medical Sciences, Rishikesh, Uttarakhand, India
| Date of Submission | 13-May-2021 |
| Date of Decision | 18-Aug-2021 |
| Date of Acceptance | 26-Aug-2021 |
| Date of Web Publication | 07-Oct-2021 |
Correspondence Address:
Shalinee Rao
Department of Pathology and Laboratory Medicine, All India Institute of Medical Sciences, Rishikesh – 249 203, Uttarakhand
India.
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.jcar_14_21
Abstract
INTRODUCTION: Focused studies in different geographic regions would delineate the underlying biological differences and molecular alterations in non-small cell lung cancer (NSCLC) worldwide. Previous studies in literature have documented limited characterization by studying a minimal number of biological markers. This study was done to evaluate expression of multiple immunomarkers including diagnostic, prognostic, and predictive markers in NSCLC for its characterization.
MATERIALS AND METHODS: This was an observational study conducted on 60 consecutive cases of NSCLC. Immunomarkers comprising of p63, p40, TTF-1, napsin A, B-Raf, c-Met, phospho-AKT (P-AKT), PTEN, anaplastic lymphoma kinase (ALK), epidermal growth factor receptor (EGFR) and K-Ras, synaptophysin, chromogranin and pan-cytokeratin were evaluated on paraffin-embedded tissue sections of NSCLC.
RESULTS: Age of patients with NSCLC in our study ranged from 35 to 90 years, and 93.3% of them were chronic smokers. 93.3% of cases presented in late stages (Stages III and IV) and 78% of cases were squamous cell carcinoma (SCC). EGFR positivity was noted in 83.3% of cases. ALK was positive in one case while C-Met and PTEN immunopositivity was noted in only two cases. Ten cases showed positivity for K-Ras and 90% of these were SCC. Ten cases were positive for B-Raf and 80% of these were SCC. 30% of cases showed immunopositivity for P-AKT. None of the molecular markers was found to have statistically significant correlation with clinicopathological parameters.
CONCLUSION: SCC is the predominant histological subtype of NSCLC in the region of Uttarakhand, India, with a high proportion of cases harboring EGFR mutation. Variable expression of K-Ras, P-AKT, ALK 1, and PTEN in NSCLC signifies that molecular profile of every case is individualistic and independent. We attribute this to ethnicity, influence of implicated substance or metabolite in tobacco, and variable mutations incurred in tumor cells over a period of time.
Keywords: Characterization, immunoexpression, molecular profile, non-small cell lung cancer.
| How to cite this article: Rao S, Anthony ML, Chowdhury N, Kathrotia R, Mishra M, Naithani M, Sindhwani G, Singh N. Molecular characterization of lung carcinomas: A study on diagnostic, predictive, and prognostic markers using immunohistochemical analysis at a tertiary care center in Uttarakhand, India. J Carcinog 2021;20:17 |
| How to cite this URL: Rao S, Anthony ML, Chowdhury N, Kathrotia R, Mishra M, Naithani M, Sindhwani G, Singh N. Molecular characterization of lung carcinomas: A study on diagnostic, predictive, and prognostic markers using immunohistochemical analysis at a tertiary care center in Uttarakhand, India. J Carcinog [serial online] 2021 [cited 2021 Oct 14];20:17. Available from: https://carcinogenesis.com/text.asp?2021/20/1/17/327604 |
Introduction
Primary lung cancer is the foremost cause of cancer-related deaths worldwide. It accounts for about 19% of cancer-related death across the world and for 9.3% of cancer-related deaths in India.[1] The pathology and epidemiology of lung cancer in a population are highly influenced by the ethnicity, geographic location, and personal habits like smoking.[1],[2] The genetic heterogeneity of lung cancer in different ethnic groups has prompted researchers toward molecular characterization in their regional population. Indian data on such molecular characterization are presently limited, possibly due to resource limitation.
There has been a major shift in the distribution of histological subtypes of non-small cell lung cancer (NSCLC) among men, women, and nonsmokers, with adenocarcinoma becoming the leading subtype. The Indian picture on this trend is still not clear due to limited epidemiological data.[2] The recent classification of lung cancer as proposed by the IASLC/ATS/ERS in 2015 based on small biopsies and cytological samples stresses on the need of histochemical stains (mucin) and immunohistochemical markers, as well as molecular studies in lung cancer.[3]
Literature search revealed only one study on lung cancer pathology from Uttarakhand.[4] Studies on immunoprofiling for subtyping and assessment of molecular predictive and prognostic markers in this population are lacking. Comparison of our study findings with available data from the rest of the world may highlight differences (if any) with respect to ethnicity. Finding such patterns of expression would be helpful in highlighting specific areas for further research and also help in the planning of treatment by providing baseline molecular data.
The Indian studies on molecular profiling are based on polymerase chain reaction (PCR), immunohistochemistry (IHC), and fluorescence in situ hybridization (FISH) testing of few (1–3) markers.[5],[6],[7],[8],[9] The aim of the present study was to evaluate the immunoexpression of multiple proteins implicated in molecular genetics of NSCLC for multi-oncoprofiling. This was done to comprehensively characterize the diagnostic, predictive, and prognostic aspects of lung cancer diagnosed in a tertiary care center in Uttarakhand, India, and to correlate the expression of these molecular markers with each other and with clinicopathological parameters such as cigarette smoking, histological subtype, stage of disease, and radiological findings.
Materials and Methods
The study was started after prior approval from the Institutional Ethics Committee, for using paraffin-embedded tissue samples (Study/Protocol No. 31/IM/2016 dated April 02, 2016). This was an observational cross-sectional study conducted at a tertiary care center in Uttarakhand, North India. Sixty consecutive cases of NSCLC which were histologically proven on core biopsy samples with sufficient tumor tissue were included in this study. Clinical details were recorded from the case sheets, and radioimaging findings from computerized tomography (CT) scan and magnetic resonance imaging (MRI) were obtained from the Radiology Department. Disease staging was done for cases as per the tumor, lymph node, metastasis (TNM) staging based on the Union for International Cancer Control/American Joint Committee on Cancer 8th Edition 2017 recommendations.[10] Hematoxylin and eosin stained tissue sections were reviewed to select 60 consecutive cases as per the inclusion/exclusion criteria. Patients who had already received either chemotherapy or radiotherapy before the core biopsy, and patients with recurrent disease were excluded from the study. Core biopsies with insufficient tissue to study up to 14 immunomarkers were also excluded from the study.
Four-micron thick paraffin embedded tissue sections were taken on poly-L lysine coated slides. Immunohistochemical staining with appropriate positive controls was done on tissue sections which were treated with the following panel of primary antibodies: p63 (Clone: 4A4; Isotype: Mouse IgG2α/, PathnSitu); p40 (Clone: ZR8; Isotype: Rabbit IgG, Master diagnostica); TTF-1 (Clone: EP229; Isotype: Rabbit IgG, PathnSitu); Napsin A (Clone: EP205; Isotype: Rabbit IgG, PathnSitu); B-Raf (Clone: 1B12; Isotype: Rabbit IgG, Biospes); c-Met (Clone: 2B3; Isotype: Rabbit IgG, Biospes); phospho-AKT (P-AKT) (Clone: Polyclonal; Isotype: Rabbit IgG, Biospes); PTEN (Clone: 6H2.1; Isotype: Mouse IgG2a, Bio SB); anaplastic lymphoma kinase (ALK) (Clone: OT1A4; Isotype: Mouse IgG2b, PathnSitu); epidermal growth factor receptor (EGFR) (Clone: EP22; Isotype: Rabbit IgG, PathnSitu); K- Ras (Clone: Polyclonal; Isotype: Rabbit IgG, Medaysis); synaptophysin and chromogranin. Pan cytokeratin was done only for cases negative for p63, TTF-1, napsin, p40, synaptophysin, and chromogranin.
Immunostained sections were independently studied by two pathologists, focusing under low power (×10) and high power (×20 and ×40) fields to detect immunoreactivity. Observations made independently were then compared, and any disagreements were resolved by common consensus. An initial histopathological diagnosis of NSCLC and subtyping was performed based on immunomarkers such as TTF-1, napsin, p63, p40, synaptophysin, and chromogranin. Cases diagnosed as NSCLC were further subjected to other IHC markers included in the panel of the study. The association of these markers with size (based on CT scan/MRI reports), age, gender, histological type, grade, clinical stage, and smoking status was estimated.
Statistical analysis
The association between findings observed with IHC markers, histological type, grade, gender, site, and clinical stage was analyzed by Fisher’s exact test. For association of the IHC markers with size, Mann–Whitney test was used. The significance level was set at P = 0.05.
Results
The age of patients with NSCLC in our study ranged from 35 to 90 years with mean age of 58.4 years [Table 1]. About 45% of cases were below 55 years of age. Male-to-female ratio was 5.7:1. 93.3% of cases were chronic smokers while 6.7% were nonsmokers. The patients belonged to Uttarakhand and Western Uttar Pradesh. About 93.3% of cases presented in late stage (Stages III and IV). Predominant histological subtype was found to be squamous cell carcinoma (SCC) comprising 78% of the cases, followed by adenocarcinoma with 20% cases [Table 1].
 |
Table 1: Distribution of cases based on clinical characteristics and positivity for various immunomarkers Click here to view |
The data on size of tumor was not normally distributed, therefore, data were summarized as median (interquartile range) and not as mean ± standard deviation. Median size of SCC was found to be 5.15 cm (range: 2–12.6 cm) and its median volume was 48.64 cm3 (range: 4–1080 cm3) in CT images [Table 2]. Median size of adenocarcinoma was 5.65 (range: 3.3–9 cm) and its median volume was 76.6 cm3 (range: 8.58–501.5 cm3) in CT images [Table 2].
| Table 2: Size of tumor based on largest dimension in computerized tomography images Click here to view |
There were 43 cases, which were positive for p63 and therefore categorized as SCC. There were four cases, which were negative for p63 but showed positivity with p40, and hence, these were also categorized as SCC. Among 12 adenocarcinoma identified by immunostains, nine of them showed positivity with TTF-1, while another three cases of adenocarcinoma were negative for TTF-1 but positive for napsin 1. One case was negative for TTF-1, napsin, p63, and p40 but positive for Pan-CK and hence was categorized as non-small cell lung carcinoma, not otherwise specified (NSCLC-NOS) [Table 3].
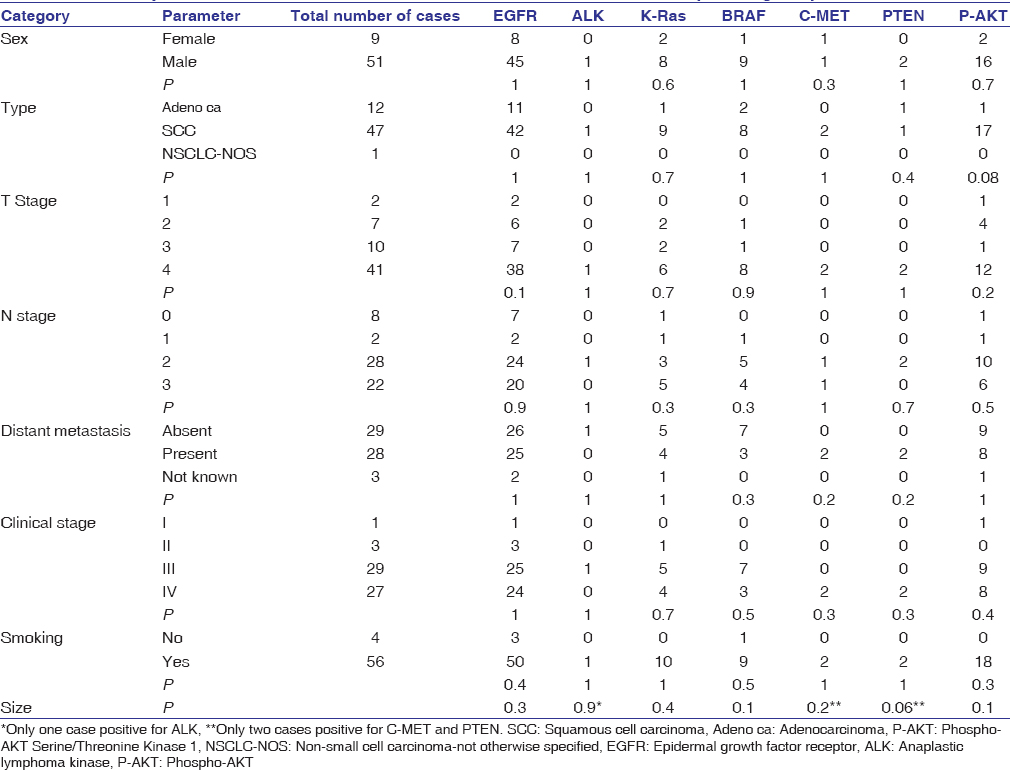 |
Table 3: Summary of results for the association of all markers with the clinicopathological parameters Click here to view |
EGFR positivity was seen in 53 cases, out of which five were 1+, 18 were 2+, and 30 were 3 + in staining intensity. EGFR positivity was noted in 83.3% cases and these were predominantly of SCC histological subtype (79.2%). ALK was positive in only one case of SCC. K-Ras was positive in 10 cases and 90% of these cases were SCC. Ten cases showed positivity for KRAS and 90% of these were SCC. There were 10 cases with positivity for BRAF and 80% of these were SCC. C-Met positivity was noted in only two cases of SCC, and PTEN immunopositivity was seen in two cases comprising of one each of adenocarcinoma and SCC. There were 30% of cases which showed immunopositivity for P-AKT and all were SCC except for one case of adenocarcinoma and one NSCLC, NOS [Table 3].
On multivariate analysis of immunoexpression of these immunomarkers with each other and various clinicopathological parameters, none of the molecular expression results were found to be statistically significant [Table 3]. The clinicopathological profile of our studied sample is given in [Table 1]. The distribution of the molecular IHC markers among the various clinicopathological groups is given in [Table 3]. None of the molecular markers showed statistically significant correlation with any particular clinicopathological parameters [Table 3].
Discussion
Molecular characterization of lung cancer needs to be explored in various ethnic groups and populations since genetic heterogeneity has been noted in various ethnic populations. This genetic heterogeneity and variations have been documented between countries, as well as within different ethnic regions of a country.[8] The exact Indian data on genetic and molecular heterogeneity in lung cancer is sparse.[2] The age of the patients in our study was lower than that found in other studies, while the percentage of smokers was higher. This possibly reflects that the population is not aware of the ill effects of smoking and that the incidence of smoking is high in this geographical/hilly region.
The use of p63, p40, cytokeratin5/6, NTRK1, and NTRK2 helps in identifying SCC while TTF-1, napsin 1, and cytokeratin 7 recognize adenocarcinoma.[3],[9] In our study, few cases of SCC were negative for p63 but positive for p40 and few adenocarcinomas were negative for TTF-1 but positive for napsin-1. Our findings highlight the importance of immunomarkers in resolving the dilemma on the nature of histological subtype for NSCLC as SCC/adenocarcinoma/NSCLC-NOS. Appropriate use of IHC definitely improves the histological subtyping and should be used as per case.
Adenocarcinoma of lung is the predominant histological pattern in the western world and most Asian countries, and has gradually overtaken SCC over the last few decades. This has been attributed to reduction in cigarette smoking and change in smoking habits with people switching to filtered cigarettes.[1] The results of our study are in contrast to this well-documented finding. Our study revealed that the histopathological patterns of lung cancer in the population of Uttarakhand and North Western Uttar Pradesh have remained unchanged, with SCC still being the predominant subtype of lung cancer in this region.[4] One possible explanation for this finding could be the fact that 93.3% of cases in our study were chronic cigarette smokers. Many studies from North India have also reported SCC to be the most common histological subtype, while occasional reports from South India have shown a change in the histological pattern, which is similar to the western world.[1],[9],[11],[12],[13]
The primary lung adenocarcinomas and SCC can be further characterized on the basis of performing a mutational profiling by molecular testing.[14] Personalized therapy in NSCLC based on genetic profiling and directing treatment against it is being practiced in many parts of the world. It is essential to have data on molecular mechanisms involved in lung carcinogenesis specific to a geographic region, and the potential therapeutic targets, which can be adopted against it. This will result in availability of molecular prognostic and predictive markers which contribute toward therapeutic decision making and raise the standard of patient care.
EGFR denotes a family of transmembrane tyrosine kinase growth factor receptors involved in a wide range of cellular processes which includes proliferation, apoptosis, and angiogenesis. The presence of mutated EGFR in NSCLC predicts high response rate to specific EGFR tyrosine kinase inhibitor (TKI) therapy, which has now become the first line of treatment in cases of NSCLC harboring EGFR mutation and advanced also in NSCLC cases.[15],[16] EGFR mutation has been associated with adenocarcinoma, nonsmokers, and Asian ethnicity, and these cases respond quite well to TKI therapy, with improvement in overall survival.[16] Literature documents that the prevalence of specific mutations may vary in various ethnic groups and geographical populations.[1] The overall percentage of NSCLC with overexpression of EGFR has been found to be 10% in Caucasians, while Asians show a higher percentage ranging from 40% to 60%.[16],[17] As per review of literature, Indian studies have documented EGFR mutation ranging from 25% to 50% in NSCLC.[1] Some studies have reported mutant EGFR in NSCLC ranging from 43% to 83% and the predominant histopathological subtype to be SCC (approximately 70%), while adenocarcinoma accounts for approximately 50% cases.[18],[19] A study by Rosell et al. documents 80.9% EGFR positivity in adenocarcinoma, 69.7% in women, and 66.6% in nonsmokers.[15]
In the present study, 53/60 cases (83.3%) showed EGFR positivity which ranged from 1 + to 3 + scores. IHC scores of 1 + to 3 + delineate NSCLC into clinically relevant high and low EGFR groups.[20] In our study, 11/12 adenocarcinoma and 42/47 of SCC were EGFR positive by IHC. A study from South India revealed immunopositivity for EGFR in 89% of adenocarcinomas, a value similar to ours.[9] Expression of EGFR documented from India showed variable findings.[14] This may possibly be influenced by ethnicity, geographic location, role play of implicated substance in cigarette, the method of detection FISH/PCR/IHC, and clone tested in IHC studies. Brevet et al. evaluated two monoclonal antibodies for the detection of EGFR mutations by IHC and found that L858R mutant antibody had a sensitivity of 95% and a positive predictive value of 99% for 1 + positivity.[21] They further concluded that EGFR mutant-specific antibody testing can be used for screening and to recognize candidates with EGFR mutation, which further can be subjected to PCR testing for typing the exon mutation.[21]
PCR is presently the gold standard to evaluate EGFR status. EGFR amplification of Exon 19 p. L861Q, p. G719X, p. S7681 responds to erlotinib, afatinib, gefitinib. EGFR amplification of Exon 20 insertion and p. T790M shows resistance to TKI, while Exon 20 p. A763_Y764 ins FQEA and Exon 21 p.L.858R point mutation respond to TKI. It has been documented that patients with lung tumors responsive to TKI tend to survive significantly longer with EGFR-TKI therapy than with only conventional chemotherapy.[15] Despite EGFR overexpression in lung tumors, there has been a reported lack of response to TKI, and this could be because of its association with several other gene mutations such as K-Ras, HER2, B-Raf, PI3K, LKB1, and SHP2. This highlights the significance of evaluating mutational analysis of the other genes to improve patient selection for TKI therapy against EGFR.[9]
In NSCLC, K-Ras mutations occur primarily in adenocarcinoma and feature exclusively in smokers. K-Ras mutation is a prognostic marker and is associated with poorer outcome. In our study, 10/60 cases showed membranous positivity for K-Ras with no statistically significant association with any clinical parameters. K-Ras mutations rarely occur with EGFR mutations and their presence has been associated with relative resistance to EGFR TKI therapy, although currently patients with K-Ras mutations are not deprived of EGFR TKI therapy.
Only 1/60 (1.6%) case in our study was positive for ALK by IHC. The ALK gene mutation/re-arrangement has been found in 3%–5% of primary lung adenocarcinomas in other studies.[22] A study by Singh and Rohtagi conducted in Northern India detected ALK mutation in 2.1% of NSCLC.[8] FISH using break-apart probes is currently the “gold standard” for the detection of ALK rearrangement, which may be seen as separation of the 5′ and 3′ FISH probes or as deletion of 5′ probe. Detection of ALK protein overexpression by IHC is highly sensitive and specific for an ALK gene rearrangement in lung adenocarcinoma using the 5A4 or D5F3 clones.[23] Recently, the Food and Drug Administration (FDA) has approved the use of Ventana ALK (D5F3) CDx assay as a companion diagnostic for crizotinib.[24] The current guidelines convey that other carefully validated, non-FDA approved, ALK IHC assays may also be used to screen for ALK rearrangement with confirmation by FISH before initiating ALK-targeted therapy.
MNNG HOS transforming (MET) gene amplification otherwise known as hepatocyte growth factor receptor may be a poor prognostic factor in NSCLC. Review of literature revealed that evidence generated through preclinical and clinical studies highlights the role of MET activation to be both a primary oncogenic driver in subsets of lung cancer and also as a secondary driver of acquired resistance to targeted therapy in other genomic subsets.[25] Mutations in the splice site of MET occur in small proportion of NSCLC, amounting to about 3%–4% of adenocarcinomas, 2% in SCCs, and approximately 1%–8% in other subtypes of lung cancer.[26] Clinical trials focused on MET pathway-directed targeted therapy have shown variable results.[25] Saigi et al. in a study on 157 NSCLCs detected METex 14 mutations and MET amplification in seven tumors; mainly adenocarcinoma and sarcomatoid carcinomas.[7] Saigi et al. also noted low level of MET protein expression in METex 14 mutated tumors and discouraged the use of MET immunostaining as an IHC marker for selecting patients for MET-targeted therapies.[7] Strong phospho-S6 immunostaining was noted in half of the MET-activated tumors.[7] Positivity for c-Met was noted in only 3.3% of our cases, all of which were histologically and IHC proven SCC. A study done by Guo et al. documented that MET gene mutation can be missed by IHC, and hence, they concluded that IHC is not an efficient tool to evaluate genomic changes related to MET amplification, which can be accurately detected by FISH or a multiplex NGS panel.[27]
Aberrant AKT activation contributes to lung carcinogenesis. AKT activation in cancer has been evaluated using phospho-specific antibodies against S473 in immunohistochemical analyses of tumor specimens.[28] Although phosphorylation of AKT at S473 has been correlated with poor clinical outcomes in many tumor types, results in lung cancer are apparently inconsistent, having been associated with either poor or good prognosis.[28] In our study, expression of deregulated PI3K pathway was assessed using IHC for mutant protein P-AKT. We found 18 of 60 cases (17 SCC and one adenocarcinoma) showing P-AKT immunoexpression. Only two out of 60 cases in our study cases showed PTEN immunoexpression, which suggests loss of PTEN in the rest (58 out of 60) of the cases in our study. PTEN negatively regulates PI3K pathway and acts as a direct antagonist of PI3K action through dephosphorylation of phosphatidylinositol (3, 4, 5)-trisphosphate. Loss of PTEN in tumor cells result in hyperactivity of PI3K signaling and tumorigenesis. In tumors with loss of PTEN, there may be overactivation of AKT caused by loss of function of PTEN lipid phosphatase.[29]
B-Raf mutations have been reported in less than 5% of NSCLC, most of which are adenocarcinomas and occur in the elderly.[5],[30] In contrast to this, 16.7% cases (10/60) in the present study showed positivity for B-Raf, eight of which were SCCs and two adenocarcinomas. All our B-Raf-positive cases were chronic smokers and their age ranged from 35 to 90 years (median age: 55 years). The probable reason for this contrasting finding in age could either be because of differing molecular genetics in this ethnic population or due to differing IHC done and warrants further investigation.
The TNM staging system for lung cancer using the 8th edition was followed for clinical staging in our study.[10] The majority of the cases (85%) were in T3 and T4 category. The lymph node status ranged from N1 to N3 (87%) detected radiologically, with distant metastasis in 46.7% at an initial diagnosis. Overall staging majority of cases presented in a high stage with 93.3% (Stages III and IV). Similar to our findings, another study conducted in Uttarakhand by Rawat et al. also showed a high percentage of cases in late Stages (III B and IV) of the disease.[4]
The data available in literature shows genetic heterogeneity among different ethnic populations, and this mandates the need for molecular characterizations of primary lung cancers by performing regional studies.[1] The published data on molecular characterization of lung cancers in Indian population is limited and lacks clarity. The present study uniquely documents the expression of various molecular markers in each case. It needs to be emphasized that there is a lot beyond morphology, which needs to be unmasked to be able to provide proper therapy and follow-up to the patients. In the last two decades, many molecular targets and newer driver mutations involved in the pathogenesis of lung cancer have been revealed, which can be amenable to targeted therapy. In our study, characterization of NSCLC based on various prognostic and predictive markers revealed that each of these are independent markers. Possibly, mutations incurred in tumor cells occur gradually over a period, which add up. The other reason could be the influence of implicated substance or metabolite causing variability in the molecular profile noted. Results of our study re-emphasize the need for individualized treatment modalities. Since molecular profiling of each of these cases is different, hence, we reiterate that it is essential for each case to be studied for pertinent predictive and prognostic marker as a part of management protocol in personalized medicine, and a generalized approach should be avoided.
Limitations of the study
This study was primarily IHC based and no further analysis was done through molecular studies such as PCR or FISH. Patients could not be followed up hence the response to treatment could not be assessed.
Conclusion
SCC is the predominant histological subtype of NSCLC in the region of Uttarakhand, with a high proportion of cases harboring EGFR mutation. In view of variability in expression for diagnostic markers, all NSCLC should be evaluated for all the four markers (TTF-1, napsin, p40, and p63) before labeling it as NSCLC-NOS. A variable expression of K-Ras, P-AKT, ALK, B-Raf, c-Met, and PTEN in NSCLC signifies that the molecular profile of every case is individualistic and independent. NSCLC is characterized with unique mutations and cannot be generalized in larger context. Findings of our study further propose that clinical behavior of NSCLC may be influenced by variability and changes in genetic, epigenetic, and phenotype resulting in different outcome and variable response to therapy among patients.
Financial support and sponsorship
The study was funded by the Institutional intramural fund grant sanctioned by the All India Institute of Medical Sciences, Rishikesh.
Conflicts of interest
There are no conflicts of interest.
References
| 1. |
Malik PS, Raina V. Lung cancer: Prevalent trends and emerging concepts. Indian J Med Res 2015;141:5-7.
 [PUBMED] [Full text] |
| 2. |
Noronha V, Pinninti R, Patil VM, Joshi A, Prabhash K. Lung cancer in the Indian subcontinent. South Asian J Cancer 2016;5:95-103.
 [PUBMED] [Full text] |
| 3. | |
| 4. |
Rawat J, Sindhwani G, Gaur D, Dua R, Saini S. Clinico-pathological profile of lung cancer in Uttarakhand. Lung India 2009;26:74-6.
 [PUBMED] [Full text] |
| 5. | |
| 6. | |
| 7. | |
| 8. |
Singh R, Rohtagi N. Clinicopathological and molecular epidemiological study of lung cancer patients seen at a tertiary care hospital in Northern India. South Asian J Cancer 2017;6:171-5.
 [PUBMED] [Full text] |
| 9. |
Shankar S, Thanasekaran V, Dhanasekar T, Duvooru P. Clinicopathological and immunohistochemical profile of non-small cell lung carcinoma in a tertiary care medical centre in South India. Lung India 2014;31:23-8.
 [PUBMED] [Full text] |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. |
Martínez-Navarro EM, Rebollo J, González-Manzano R, Sureda M, Evgenyeva E, Valenzuela B, et al. Epidermal growth factor receptor (EGFR) mutations in a series of non-small-cell lung cancer (NSCLC) patients and response rate to EGFR-specific tyrosine kinase inhibitors (TKIs). Clin Transl Oncol 2011;13:812-8.
 |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. | |
| 25. | |
| 26. | |
| 27. | |
| 28. | |
| 29. | |
| 30. |
