Bhavna Daswani, Yasmin Khan
Department of Life Sciences, Sophia College (Autonomous), Mumbai, Maharashtra, India
| Date of Submission | 24-Jan-2021 |
| Date of Decision | 19-Feb-2021 |
| Date of Acceptance | 24-Apr-2021 |
| Date of Web Publication | 13-Aug-2021 |
Correspondence Address:
Bhavna Daswani
Department of Life Sciences, Sophia College (Autonomous), Mumbai – 400 026, Maharashtra
India.
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_2_21
Abstract
Gliomas are more common in males than in females. Emerging evidence from several studies in vitro and in vivo have shown the role of estrogens and androgens in glial tumorigenesis. In recent times, studies have also shed light on the actions of estrogen receptors, alpha and beta, and androgen receptor. Here, we provide a comprehensive overview of the research hitherto on estrogens and androgens along with an emphasis on their receptors in glioma pathophysiology. Studies with conflicting results are discussed and future possibilities are put forward. A collective understanding of the studies on these steroid hormones in glioma may serve to create an amalgamated therapeutic approach; and thereby, augment the efforts in tackling this deadly disease.
Keywords: Androgen, estrogen, glioblastoma, glioma, hormones.
| How to cite this article: Daswani B, Khan Y. Insights into the role of estrogens and androgens in glial tumorigenesis. J Carcinog 2021;20:10 |
| How to cite this URL: Daswani B, Khan Y. Insights into the role of estrogens and androgens in glial tumorigenesis. J Carcinog [serial online] 2021 [cited 2021 Oct 14];20:10. Available from: https://carcinogenesis.com/text.asp?2021/20/1/10/323800 |
Introduction
Gliomas are the most common and challenging oncological manifestations arising from glia or glial stem cells of the central nervous system.[1] Conventional modes of treatment comprise of surgical removal (if feasible), radiotherapy, and/or chemotherapy/(targeted therapy/immunotherapy), depending upon the WHO grades.[2] Sadly, low-grade gliomas often progress into higher grades. Prognosis is poor for Grade IV (glioblastoma multiforme [GBM]), a deadly malignant form of glioma, the median survival time being approximately 12–15 months even after intensive treatment.[3],[4] At present, due to multimodal treatment strategies, the 2-year overall survival rate seems to be 18%, which is yet seemingly small.[5],[6] Despite significant strides in research internationally, the etiology of glial tumorigenesis is not entirely understood, and it may be governed by multiple pathways.[7],[8] Therefore, a continual search for new molecules and pathways is warranted to expand our horizons on the nature of this deadly disease, and consequently, aid in the identification of more effective therapies.
Intriguingly, it was found that gliomas are more common in males than in females.[9],[10],[11],[12],[13] In a retrospective study on gender and survival, it was found that females may possess slightly higher cancer-specific survival (in univariate analyses).[14] In congruence, recent meta-analysis studies show that women who have used exogenous estrogen (either oral contraceptives or hormone replacement therapy) have lower risk of glioma compared to nonusers.[15],[16] It is known that steroid hormones can easily pass through the blood–brain barrier.[17] Moreover, enzymes responsible for the synthesis of estrogens and androgens have also been detected in GBM cells, along with the expression of their respective receptors.[18],[19],[20],[21],[22],[23] Interestingly, estrogen and androgen receptors have been detected in normal glial cells as well as in glioma cells.[18],[19],[22],[23] Pertinently, efforts have been directed toward understanding the relationship between sex steroids and glioma. Here, we summarize the reports on the role and possible mechanisms of estrogens, androgens, and their respective receptors, in the pathophysiology of gliomas. Studies on serum/tissue levels of estrogen and androgen in relation to glioma are also summated. Overall, this is an up-to-date and comprehensive review aiming to help researchers keep abreast with the existing knowledge on the role of sex hormones (androgen and estrogen) in glial tumorigenesis.
Role of Androgens in Glioma
Androgens are steroid hormones (including testosterone and its more potent metabolite dihydrotestosterone [DHT]) that act via binding to the androgen receptor (AR). This results in the dissociation of AR from heat shock proteins leading to its transportation into the nucleus wherein its dimers bind to AR elements in the promoters of target genes to induce their transcription. Another important androgen, dehydroepiandrosterone (DHEA), is a precursor of testosterone and is primarily produced by the adrenal glands (and by the testis in very small amounts). Although DHEA binds to AR with low affinity, it is an important neurosteroid as it modulates many functions in the brain where it is also synthesized.
One of the initial attempts to understand the gender bias in glioma was conducted in athymic nude mice wherein human primary glioma was transplanted in male and female mice.[24] Remarkably, males showed significantly larger tumors, lower latency periods, and ARs were observed preferentially in the male rather than in female tumors.[24] In the last decade or so, many studies have improved our understanding on the role of androgens in glioma by exploring the effects of natural androgens and synthetic androgen agonists/antagonists in vitro and in vivo. Several studies have also analyzed the expression of AR in patient biopsy samples [listed in [Table 1]].
 |
Table 1: List of studies showing androgen receptor expression in glioma biopsy samples Click here to view |
Apart from this, AR expression has been confirmed in various glioma cell lines (A172, LN18, LN229, M059, T98G, U87, U118MG, U138MG, and U251MG).[22],[25],[26],[27],[28],[29],[30]
Studies on natural androgens and synthetic androgen agonists
Yu et al. studied the in vitro effects of DHT on human U87 GBM cells wherein they found that DHT alone had no effect[25] and this was also later confirmed by Zalcman et al.[27] However, DHT when administered in combination with transforming growth factor beta (TGF-β) decreased the latter’s antitumorigenic effect on growth and apoptosis.[25] DHT interfered with TGF-β signaling by the binding of phosphorylated AR to phosphorylated SMAD3 (which acts downstream to TGF-β), thereby preventing SMAD3 from executing its function as a transcription factor, and thus, they hypothesized that the pro-tumorigenic effect of DHT could be via inhibiting the TGF-β pathway.[25] Interestingly, Rodríguez-Lozano et al. recently showed that DHT had an independent and stronger effect than testosterone toward enhancing cell growth, proliferation, migration, and invasion in U87 and U251 cells in vitro.[30] These results were also confirmed by Orozco et al. wherein it was shown that DHT (not androstenedione) could independently increase cell viability, proliferation, and invasion in human U87 GBM cells in vitro in a more potent fashion than testosterone.[31] In fact, Rodríguez-Lozano et al. also analyzed RNA-sequencing (RNA Seq) data retrieved from the TCGA database and found that one of the enzymes responsible for metabolizing testosterone to DHT (SRD5A2 or 5 alpha reductase 2) was upregulated in low grade glioma and GBM compared to normal brain tissue.[30] Regarding testosterone, an initial study had shown that exposure of human A172 GBM cell line to testosterone decreased cell viability at higher concentrations, but had no effect at lower concentrations.[32] In contrast, another study showed that testosterone increased proliferation, cell migration, and invasion at 100nM (not at 1nM or 1000nM) in three human GBM cell lines (D54, U87, U251) in vitro.[22] As mentioned earlier, Rodríguez-Lozano et al. and Orozco et al. confirmed that testosterone indeed increased cell viability, proliferation, migration, and invasion, though not as potently as DHT.[30],[31]
A synthetic androgen agonist (R1881 or methyltrienolone) was investigated for its in vitro effects on two human GBM cell lines U87 (which expresses wild type p53) and U251 (which expressed mutant p53).[26] Interestingly, R1881 resulted in downregulation of a protein called SVIP (small VCP/p97-interacting protein expression) which in turn lead to increased cell proliferation and reduction in levels of p53 in U87 cell line, while this was not seen in the U251 cell line.[26] This meant that AR signaling may be related to a reduction in SVIP leading to the reduction of p53 and thus increased cell proliferation in cells expressing wild type p53.[26]
Initially, a study on DHEA showed that it prevents cell death in rat C6 GBM cells induced by glucose deprivation.[33] Successively, three recent studies have shed light on the role of DHEA in glioma and also its ability to diminish the inhibitory effects of temozolomide (TMZ), the drug usually used in GBM treatment. Chuang and colleagues conducted an extensive study on CYP17A1 gene which encodes for the lyase enzyme that catalyzes the production of DHEA.[34] Interestingly, they found that specificity protein 1 (Sp1; a transcription factor known for driving the expression of CYP19 and CYP11A1) was found to play a role in driving CYP17A1 expression.[34] Using a combination of bioinformatics tools and RNA Seq databases, they found that both CYP17A1 mRNA and Sp1 mRNA were significantly higher in glioma compared to normal brain tissue and correlated poorly with prognosis.[34] In vitro assays showed that CYP17A1 knockdown increased TMZ induced cytotoxicity and abolished the resistance acquired by U87-R (human TMZ-resistant GBM cells) and in these cells, there was increased binding of Sp1 to the promoter region of CYP17A1.[34] Sp1 overexpression promoted glioma invasion and knockdown abolished invasiveness and decreased DHEA secretion.[34] Further, they found that Sp1 knockdown increased DNA methylation suggesting favourable implications in O6-Methylguanine-DNA Methyltransferase dependent TMZ resistance.[34] Also, DHEA attenuated TMZ’s repressive effects on proliferation and DNA damage and inhibited TMZ induced apoptosis.[34] Collectively, the Sp1-CYP17A1-DHEA axis was shown to be a crucial player in promoting glial tumorigenesis. Ensuing this, Yang and researchers investigated the post-translational modifications on Sp1.[28] To be able to function, Sp1 needs to get phosphorylated, while acetylation aborts it function. They found that DHEA induces Sp1 phosphorylation (by activating the LYN/Akt pathway) and also its deacetylation and promotes subsequent recruitment by damaged DNA.[28] Both phosphorylation and deacetylation are significant in TMZ resistant GBM.[28] Finally, Lin and investigators found that inhibition of CYP17A1 enzyme by abiraterone (enzyme inhibitor) leads to tumor suppression in vitro and in vivo.[35] Abiraterone induced endoplasmic reticular stress and increased reactive oxygen species (to induce cell death machinery) while CYP17A1 prevented these phenomena.[35] Largely, all these studies are suggestive of the pro-tumorigenic roles of androgens including DHT, testosterone, and DHEA.
Studies on androgen receptor and androgen receptor antagonists
Bao et al. showed that AR antagonists, enzalutamide and apalutamide, significantly reduced cell proliferation in human U87 GBM cells in vitro and in an in vivo mouse tumor xenograft model; whereas, this effect was not seen in the human p53 mutant harboring U251 cells.[26] The authors suggested that p53 mutations should be checked post-surgery to influence therapeutic strategies.[26] Likewise, in a separate study by Zalcman et al., two AR inhibitors, namely, bicalutamide and enzalutamide, caused dose-dependent apoptotic cell death in three human GBM cell lines (A178G, U87, and T98G) in vitro; though, enzalutamide showed higher efficacy.[27] Furthermore, treatment with enzalutamide resulted in a substantial reduction in tumor volume in a mouse xenograft tumor model.[27] Following this, a study showed that an AR antagonist called flutamide could block the testosterone induced increase in proliferation, cell migration, and invasion of three human GBM cell lines (D54, U87, U251) in vitro.[22] Agreeably, it was recently shown that dutasteride (5 alpha reductase inhibitor), as well as cyproterone and flutamide (AR antagonists), inhibited U87 cell metabolism (viability) and proliferation.[31] Combinations of enzyme inhibitors and AR antagonists enhanced the inhibitory effects and they found that the combination of dutasteride and flutamide was most effective, while the latter also inhibited cell invasion.[31]
With regard to AR, it was shown that its knockdown correlated with tumor cell death in vitro.[29] A recent report demonstrated that overexpressing AR in human GBM U87 cells led to increased proliferation and subsequently TMZ resistance in vitro; whereas, knockdown of AR decreased proliferation.[29] They developed a compound called ALZ003 which is structurally analogous to curcumin with the property of degradation of AR protein (designated as Orphan Drug by the Food and Drug Administration [FDA]). ALZ003 downregulated AR, induced oxidative stress (ferroptosis, lipid peroxidation, and accumulation of reactive oxygen species), and inhibited the growth of U87 GBM cells with or without TMZ resistance in vitro and also in a mouse model in vivo.[29] They also used a combination of bioinformatics databases and tools (TCGA, Oncomine, and SurvExpress) to show that AR expression was significantly higher in glioblastoma than in normal brain tissue and high AR expression correlated with poor prognosis.[29] Another recent study reported that cedrol (a sequesterine alcohol that has antimicrobial and anticancer properties) inhibited the growth of GBM cells and induced apoptosis (in synergy with TMZ) in vitro as well as in vivo in mouse models.[36] Interestingly, cedrol inhibited GBM via blocking AR; this was shown using bioinformatics analyses.[36] They also showed that cedrol blocked AR nuclear translocation, reduced cell viability, and mRNA expression of AR activated genes (like KLK3 and TMPRSS2) in DHT-treated cells compared to control.[36] Overall, AR signaling seems to be pro-tumorigenic and AR antagonists have shown some efficacy in vitro and in vivo in animal models.
Role of Estrogen in Glioma
Estrogen has three biological forms, namely, estradiol, estriol, and estrone, of which estradiol (rather 17β estradiol) is the most potent and predominant form in menstruating females. Estrogen mediates its functions preferentially via two of its cognate receptors – estrogen receptors – alpha and beta (ERα and ERβ). These ERs once activated by the binding of the ligand, get dissociated from the bound heat shock protein, and get dimerized and translocated to the nucleus wherein they activate the transcription of target genes by binding to the estrogen response element in their promoter regions. It is noteworthy that ERα and ERβ regulate a shared set of target genes but also regulate different target genes which contribute to their differing roles in different diseases.[37] Estradiol can bind to both ERα and ERβ as they share structural similarities; even so, subtle differences in their ligand-binding pockets have enabled the design of specific agonists in order for us to understand their unique roles.[38],[39]
Studies on 17β-estradiol in glioma
A study by Plunkett et al. in 1999 reported a significant positive survival advantage of female compared to male rats implanted with human U87 GBM cells in vivo.[40] The progression of the tumor was slowed in the presence of exogenous or endogenous estradiol, resulting in a 20% prolongation of life.[40] Ovariectomy cancelled out the female survival advantage while an estrogen replacement restored it (this effect was not seen with progesterone replacement).[40] After almost a decade, a similar in vivo study reinforced that exogenous estradiol treatment increased survival of male and female (normal and ovariectomized) orthotopic rats, and brain sections of estradiol-treated rats displayed higher apoptotic index.[41] An in vitro study by Altiok et al. reported that a high dose of estradiol induces apoptosis and suppresses cell growth in a concentration- and time- dependent manner in C6 glioma and T98G glioblastoma cells along with activation of c-Jun N-terminal kinase signaling.[42] In agreement with this, a recent study showed that estradiol (at high concentrations) decreased cell viability and increased sensitivity to TMZ.[43] Conversely, few in vitro studies have also shown the opposite effect of estradiol. At low concentrations, estradiol increased proliferation and mitochondrial fitness of U87 cells.[44] Estradiol increased migration of T98G GBM cells and increased cell invasion in U87 GBM cells, in a concentration dependent manner ranging from low to high concentrations.[45] In another study, estradiol increased migration of C6 cells but not in F98 cells, both of which are rat GBM cell lines.[46] Further, González-Arenas et al. showed that estradiol increased cell numbers in a time-course study in grade III U373 human cells (on day 4) and Grade IV D54 human cells (on day 5).[23] This effect was blocked on coadministering ER antagonists (IC182,780).[23] More recently, an elegant study by the same group revealed that estradiol induces a partial epithelial to mesenchymal transition (EMT) in U87 and U251 cells, supported by assays on cell morphology, expression and redistribution of EMT markers, as well as increased migration and invasion.[47] Taken together, the studies on the direct influence of estradiol on glial tumorigenesis have shown varying results. However, the action of estradiol is governed by the role of its cognate receptors, namely, ERα and ERβ, which may trigger distinct downstream pathways. Hence, it is essential to understand the specific roles of ERα and ERβ in the glioma context. [Table 2] shows the list of studies on the expression of ERα and ERβ receptors in gliomas in patient biopsy samples. With regard to glioma cell lines, ERβ expression has been found in several glioma cell lines (T98G, U87, LN229, U138, MO59J, MO59K, U373, D54, C6) in many studies.[23],[46],[47],[48],[49].However, there are differing reports for ERα expression in glioma cell lines. One study showed that ERα is absent in T98G, U87, LN229, U138, MO59J, MO59K;[48] whereas, other studies show that ERα is expressed in U373 and D54,[23] and also in LN229, LN18, U251, U87, T98G cell lines.[43],[47] These differences may have arisen due to the technique/molecule (mRNA/protein) being tested and/or differences in antibodies used/passage number of the cells.
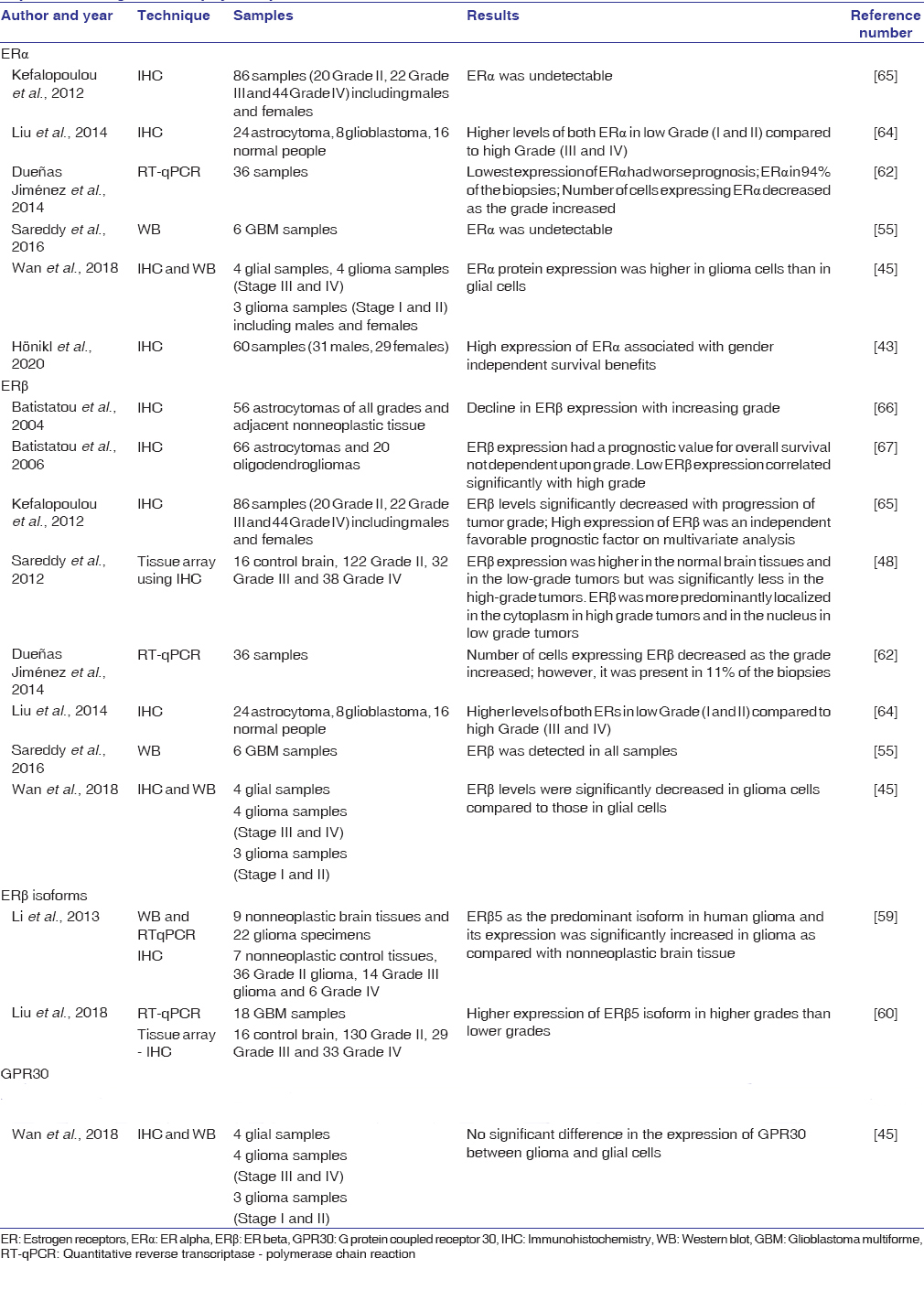 |
Table 2: List of studies showing estrogen receptors (estrogen receptor alpha and estrogen receptor beta) expression in glioma biopsy samples Click here to view |
Apart from estradiol, studies have also addressed the effect of tibolone on glioma cells. Tibolone is a synthetic steroid with tissue-specific hormonal (estrogenic/progestogenic/androgenic) activity and is used to treat menopausal symptoms and postmenopausal osteoporosis in some countries. Among tibolone’s active metabolites, 3α-hydroxy tibolone and 3β-hydroxy tibolone are responsible for estrogenic activity and have a longer half-life than the Δ4-isomer of tibolone, which has progestogenic and androgenic activities and is rapidly cleared from circulation.[50] Furthermore, the hydroxy metabolites are predominantly found in the brain of non-human primates.[51] An initial study on the effect of tibolone on glioma cells showed that tibolone had antiproliferative activities on human GBM cells from patients, whereas this effect was not seen in C6 rat GBM cells.[52] Later, another in vitro study showed that tibolone increased the number of glioma cells (U251 and U87), while it did not have any effect on cell migration and invasion.[53] They also showed that it increased the expression of both ERs and progesterone receptor.[53] Perhaps, in vivo studies on tibolone’s effects may aid in defining its effects in glioma.
Studies on estrogen receptors beta in glioma
Numerous studies indicate a protective role played by ERβ in glioma. Sareddy et al. studied the effects of liquiritigenin and MF101 (natural ERβ agonists) and diarylpropionitrile (DPN; a synthetic ERβ agonist) all of which decreased cell proliferation in human GBM cell lines (U87, LN229, T98G, U138MG), while knockdown of ERβ inhibited this effect.[48] Further, they showed that these ERβ agonists reduced colony formation and caused cell cycle arrest in U87 and LN229 GBM cells.[48] In vivo liquiritigenin resulted in a significant reduction in tumor volume as observed in mouse xenograft models using U87 GBM cells.[48] Additional analysis revealed that liquiritigenin-treated mice showed increased apoptosis, decreased proliferation, and increased ERβ expression and nuclear localization while no toxicities were seen in terms of behavioral changes.[48] Thereafter, Liu and researchers reported that liquiritigenin induced dose-dependent cell death in the U138 TMZ resistant cell line, and the effect of liquiritigenin was synergistic with TMZ (even with low dose TMZ).[49] Since ERβ overexpression is known to inhibit the PI3K/Akt/mTOR (phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin) pathway (primarily a cell survival pathway), they sought to examine this in U138MG cells by checking the protein expression and phosphorylation of AKT and P70S6K, important players in this pathway.[49] Treatment with liquiritigenin (and not TMZ) caused a decrease in phosphorylation of AKT and P70S6K, while ERβ knockdown reversed this decrease. Also, XL765 (PI3K/mTOR inhibitor) increased the sensitivity of U138 cells to TMZ, even after accounting for the effect of the latter.[49] Another report showed the novel design and synthesis of new salicylketoxime derivatives which selectively bind to ERβ and its antiproliferative effects in U87 glioma cells in vitro along with a reduction in tumor volume in a mouse model in vivo.[54] Subsequently, Sareddy et al. showed that LY500307, a synthetic ERβ agonist, decreased proliferation and colony formation, and increased apoptosis of U87 and U251 GBM cells.[55] Further, knockdown of ERβ abrogated the inhibitory effects of LY500307 while ERβ overexpression resulted in a further decrease in proliferation.[55] RNA-seq of U87 cells with or without ERβ revealed many pathways affected including cell cycle, cell death, survival, and DNA damage response.[55] As cell cycle seemed to be the most affected pathway, they also confirmed that LY500307 induced cell cycle arrest in G2/M phase in U87, U251, LN229, and GBM10 cells. LY500307 also sensitized cells to FDA approved drugs cisplatin, lomustine, TMZ.[55] To reach a successful conclusion, they studied the effect of LY500307 in two in vivo models, namely, an orthotopic and syngeneic glioma mouse model.[55] In the former, tumors are engrafted into the relevant organ as against a xenograft model wherein tumors are engrafted under the skin, whereas, the latter receives allograft tissues from the same strain and has an intact immune system enabling a more holistic view similar to that seen in human oncological conditions. For the orthotopic model, U251 cells were injected into the brain of mice and subsequent treatment with LY500307 reduced tumor burden, and assays from treated mice showed a decrease in proliferation and increase in apoptosis.[55] For the syngeneic model, GL26 cells were implanted into C57BL/6 mice and subsequent LY500307 treatment increased overall survival, increased apoptosis, and decreased proliferation markers.[55] Next, the same group of investigators recently showed that ERβ expression is present but at low levels in U87, U251, and T98G GBM cell lines.[56] Hence, they overexpressed ERβ in U87 cells and carried out RNA Seq analyses which revealed that ERβ modulated pathways were related to DNA damage check-point regulation, DNA damage response, DNA repair, ATM signaling pathways and cell cycle.[56] They validated genes for DNA damage at the mRNA level in GBM cell lines (U87, U251) and patient-derived GBM cells. Gene Set Enrichment Analysis of the differential transcriptome revealed that ERβ regulated genes were negatively correlated with homologous recombination.[56] Hence, they performed in vitro assays to establish that ERβ inhibits homologous recombination pathway by decreasing ATM expression and phosphorylation.[56] Further, they showed that ERβ enhances chemotherapy response and induces DNA damage by increasing levels of ϒH2AX foci which leads to apoptosis in vitro.[56] Lastly, using an orthotopic mouse model, it was found that U87 and T98G cells overexpressing ERβ had increased sensitivity to TMZ treatment with longer survival, and analyses of mice tissue sections showed increased apoptosis and ϒH2AX foci.[56] Strikingly, this suggests that ERβ can enhance TMZ sensitivity even in TMZ resistant cell types.
Another natural compound toosendanin (TSN) inhibited GBM cell proliferation and increased apoptosis in human U87 and rat C6 cells lines in vitro.[57] In addition, TSN reduced tumor burden in athymic nude mice transplanted with TSN pretreated U87 cells in vivo and immunohistochemistry from the nude mice showed that TSN was able to inhibit apoptosis.[57] Of note, treating these cells with TSN increased expression of ERβ and p53 and it was found that both these work in concert to mediate cytotoxicity of TSN.[57] To confirm its effects, the investigators performed knockdown of ERβ which resulted in abrogation of pro-apoptotic effects of TSN in U87 cells.[57] Further, a more recent study showed that a natural compound called icartin (prenyl flavonoid derived from a Chinese herb) inhibited proliferation of rat C6 and human U87 GBM cells in a dose-dependent and time-dependent manner, increased apoptosis, reduced migration, and reduced matrix metalloproteinases 2 and 9 in vitro.[58] Icartin’s mode of action was by increasing the expression of ERβ, and Phosphatase and tensin homolog (PTEN), thus reducing its downstream targets – Akt and pAkt.[58] Similar to the previous study on toosendanin, inhibiting ERβ with a specific antagonist attenuated the role of icartin.[58]
It is important to draw attention to the fact that ERβ has 5 isoforms and two independent studies have considered the role of these isoforms in glioma. Li et al. found that ERβ5 is predominant in human glioma specimens compared to non-neoplastic controls and in general ERβ increases due to hypoxia and Hypoxia-inducible factor signaling.[59] Both ERβ 1 and ERβ5 suppressed cell proliferation and cell cycle, increased PTEN expression, and inhibited PI3K/Akt/mTOR pathway, while ERβ5 also suppressed the MAPK/ERK (mitogen activated protein kinase/extracellular signal-regulated kinase) pathways.[59] In contrast, Liu et al. found that GBM cells predominantly express ERβ1 and ERβ5 and the latter was detected more in the higher grades.[60] In ERβ knockouts uniquely expressing ERβ1 or ERβ5, only ERβ1 significantly reduced proliferation.[60] RNA Seq analyses showed different profiles for ERβ1 and ERβ5, indicating that the former may be a tumor suppressor and the latter tumor promoter.[60] Further, a recent study showed more amount of ERβ1 compared to ERβ5 in four GBM cell lines (U251, U87, T98G, LN229).[47] To summarize, the antitumorigenic role of ERβ has been demonstrated in many studies; however, the exact role of the subtypes is yet to be fully unraveled.
Studies on estrogen receptors alpha in glioma
Evolving evidence suggests that estradiol may behave as pro-tumorigenic via ERα signaling. A study reported in 2012 showed that estradiol significantly increased proliferation in human GBM U373 and D54 cell lines and ER antagonists (IC182,780) could block these effects in vitro.[23] Intriguingly, these effects persisted on administering ERα agonist propyl-pyrazole triol (PPT) (though ERβ agonist (DPN) had no effects).[23] Interestingly, a recent study showed that estradiol increased EMT via cytoskeletal changes in cell morphology and heightened expression of mesenchymal markers and increased migration and invasion of U87 GBM cells in vitro.[47] Similar to the earlier study, epithelial to mesenchymal changes were obtained when treated with ERα agonist (PPT) but not ERβ agonist (DPN), while treatment with ER antagonist abrogated these epithelial to mesenchymal effects.[47] Further studies including mechanistic approaches may aid in reaffirming the role of ERα in glioma.
Estrogens and Androgens in Serum/Biopsy Samples
Studies on serum levels of estradiol in association with glioma are sparse and mostly inconclusive. A study on thyroid disorders in high-grade glioma patients reported that a majority of the women aged 50 years or older exhibited the postmenopausal hormone pattern with elevated serum gonadotropins and very low estradiol and progesterone; however, this was also the case for some younger women (24 out of 46 women) aged less than 50 years.[61] In another study, estradiol was estimated in 36 patient biopsies using ELISA and they found that estradiol was higher in GBM compared to lower grade gliomas.[62]
Serum testosterone tested in three categories, namely, normal cases with nervous problems (42 males and 76 females), benign brain tumors like pituitary adenomas (64 males and 112 females), and malignant/benign glioma cases (122 males and 67 females), revealed that the levels were significantly higher in glioma group than in the controls and the benign tumor group.[26] However, there was no significant difference in testosterone in the WHO grades; hence, the authors suggested that the elevation in testosterone levels might occur before tumor progression.[26] Further, although it is well known that testosterone levels gradually tend to decline with age, a study showed that among high grade glioma patients (including 436 patients of which 198 were female and 238 males aged 19–83 years with a median age of 50 years), 55% of men aged 50 years or older had high levels of testosterone.[61] Furthermore, a study on DHEA reported that serum levels were significantly higher in 26 glioma patients (14 males and 12 females; in all 4 WHO glioma grades) compared to 15 normal individuals (7 males and 8 females).[34] More studies are needed with larger sample size to ascertain a trend in serum levels of these hormones in patient samples.
Estrogens and androgens act via different pathways to influence disease pathophysiology. [Figure 1] is a schematic on the role of estrogen and androgen, and their respective receptors, in glial tumorigenesis.
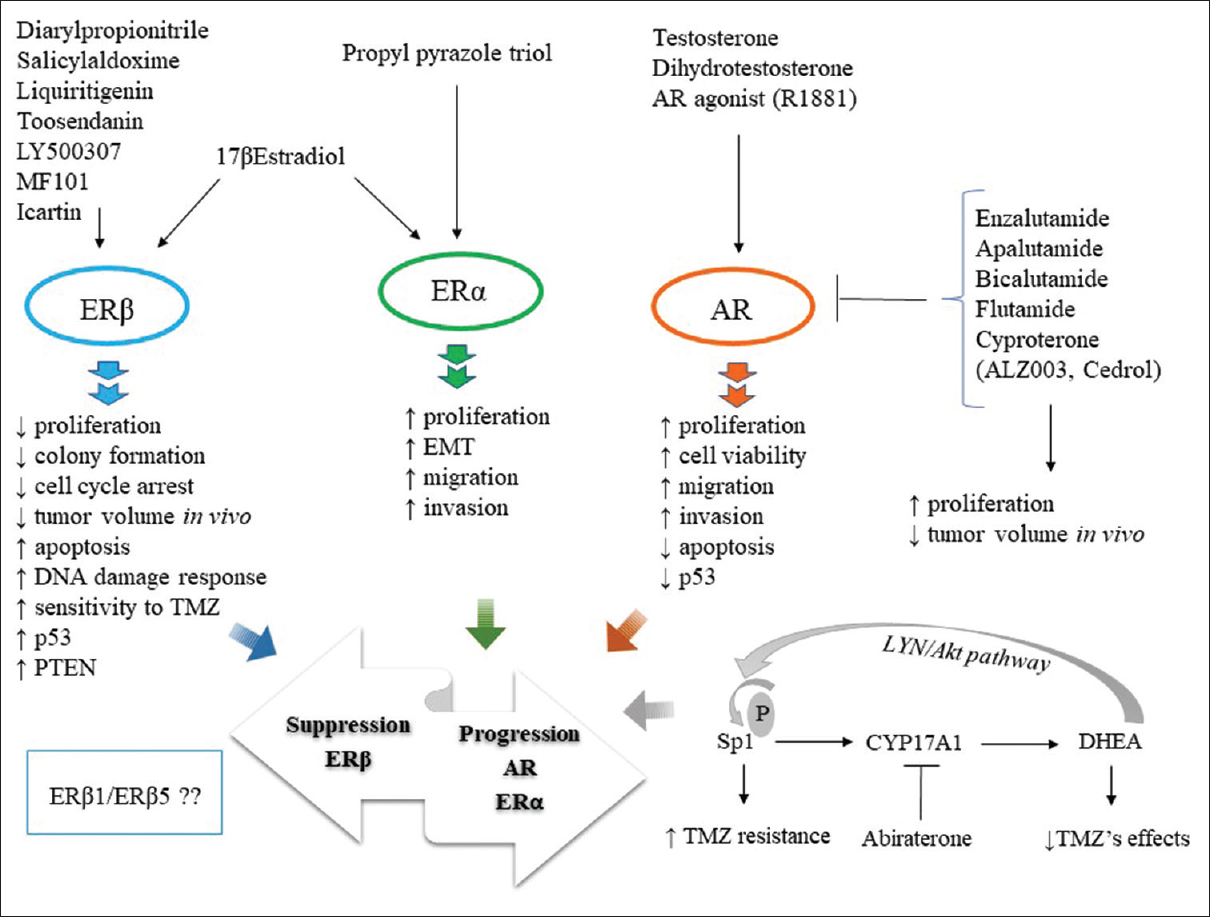 |
Figure 1: Role of estrogens and androgens and their respective receptors – estrogen receptor alpha and beta and androgen receptor, in glial tumorigenesis. estrogen receptor beta agonists shows protective effects, whereas, estrogen receptor alpha agonist appears pro-tumorigenic in glioma. Androgens and androgen receptor agonist enhance, whereas, androgen receptor antagonists seem to prove effective in controlling glial tumor burden. The bottom right shows the specificity protein 1 -CYP17A1-DHEA axis in promoting glioma progression. Overall, the activities of estrogen receptor beta, estrogen receptor alpha, and androgen receptor appear to influence the oncogenic route (progression/suppression) in glioma Click here to view |
Conclusions and Future Perspectives
The relationship between sex steroids and carcinogenesis has always been an interesting area for cancer researchers. In terms of glioma, only recently there has been a surge of reports on the role of estrogens and androgens. Gleaning from current literature, androgens and AR signaling seem pro-tumorigenic in glioma. While estradiol seems to show contrasting results in different studies, there is more clarity on the role of ERβ in glioma. Many studies are in agreement that ERβ may be antitumorigenic. In contrast, recent studies suggest that ERα may be pro-tumorigenic in glioma; however, more research is needed in this area.
Furthermore, whether up/downregulation of these receptors in increasing glioma grades is a cause or consequence is not yet known. In particular, the ratio of ERβ to ERα, and also AR, may be an interesting aspect to investigate. Mechanistic studies on the differential effects of estrogens and androgens/between ERα and ERβ may provide a better understanding of pathways triggered.[68] Besides, acquired ligand-independent activity is an important factor to consider as it occurs for both androgen signaling as well as estrogen signaling. Finally, a prospect worth exploring is a combination of ERβ agonists, AR antagonists, and androgen synthesis enzyme inhibitors possibly as adjuvant therapy, provided they cross the blood–brain barrier. These are exciting possibilities and areas for investigation; however, we need to gain deeper insights into this avenue before delving into such treatment regimens.
Financial support and sponsorship
This work is funded by Department of Science and Technology (DST), Govt. of India., under Women Scientist -A (WOS-A) scheme (ref no. SR/WOS-A/LS-475/2018). B.D. acknowledges the financial support from DST WOS-A.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. |
González-Arenas A, Hansberg-Pastor V, Hernández-Hernández OT, González-García TK, Henderson-Villalpando J, Lemus-Hernández D, et al. Estradiol increases cell growth in human astrocytoma cell lines through ERα activation and its interaction with SRC-1 and SRC-3 coactivators. Biochim Biophys Acta 2012;1823:379-86.
 |
| 24. | |
| 25. | |
| 26. | |
| 27. | |
| 28. | |
| 29. | |
| 30. | |
| 31. | |
| 32. | |
| 33. | |
| 34. | |
| 35. | |
| 36. | |
| 37. | |
| 38. | |
| 39. | |
| 40. | |
| 41. | |
| 42. | |
| 43. | |
| 44. | |
| 45. | |
| 46. | |
| 47. | |
| 48. | |
| 49. | |
| 50. | |
| 51. | |
| 52. | |
| 53. | |
| 54. | |
| 55. | |
| 56. | |
| 57. | |
| 58. | |
| 59. | |
| 60. | |
| 61. | |
| 62. | |
| 63. | |
| 64. | |
| 65. | |
| 66. | |
| 67. | |
| 68. |
Figures
[Figure 1]
