Deepak Kanojia1, Sharada S Sawant2, Anita M Borges3, Arvind D Ingle2, Milind M Vaidya2
1 Department of Biological Science, University of South Carolina, Columbia, USA
2 Advanced Centre for Treatment, Research and Education in Cancer, Tata Memorial Centre, Kharghar, Navi Mumbai, India
3 Department of Histopathology, Asian Institute of Oncology, S.L. Raheja Hospital, Mahim, Mumbai, Maharashtra, India
| Date of Submission | 21-Mar-2012 |
| Date of Acceptance | 16-Jul-2012 |
| Date of Web Publication | 13-Sep-2012 |
Correspondence Address:
Milind M Vaidya
Advanced Centre for Treatment, Research and Education in Cancer, Tata Memorial Centre, Kharghar, Navi Mumbai
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1477-3163.100861
Abstract
Background: Oral squamous cell carcinoma (OSCC) is the sixth largest group of malignancies globally and the single largest group of malignancies in the Indian subcontinent. Despite the advances in treatment and therapeutic modalities the five year survival rate of OSCC has not changed in the last few decades, and remains less than 40%. Several studies have focused on defining molecular markers that can either detect cancer at an early stage or can predict patient’s outcome. However, such markers are still undefined. Keratins (K) are epithelia predominant intermediate filament proteins which are expressed in a differentiation dependent and site specific manner. Keratins are being used as biomarkers in different epithelial disorders including cancer. They are associated with desmoplakin and α6β4 integrin which are components of desmosomes and hemidesmosomes respectively. Materials and Methods: 4-Nitroquinoline 1-Oxide (4NQO) was used as a carcinogen for the development of various stages of oral carcinogenesis in rat lingual mucosa. Two-Dimentional gel electrophoresis was performed for the separation of Keratins followed by western blotting for their specific identification. Western blotting and RT PCR was carried out for desmoplakin and α6β4 integrin respectively to understand their levels. Immunohistochemical analysis was carried out to further study the localization of desmoplakin and α6 integrin. Results: In this study we have analysed the alterations in Keratins and associated proteins during sequential stages of 4NQO induced rat oral carcinogenesis. Our results showed that the alterations primarily begin after the dysplastic changes in the lingual epithelium like the elevation of Keratins 5/6a, ectopic expression of Keratin 8, increase in suprabasal expression of α6 integrin and increase in desmoplakin levels. Most of these alterations persisted till the development of SCC except desmoplakin, the levels of which were downregulated in papillomatous lesions and SCC. Many of these alterations have also been documented in human oral carcinogensis. Conclusion: Thus, 4NQO model of rat lingual carcinogenesis reproduces majority of the changes that are seen in human oral carcinogenesis and it can be exploited for the development of biomarkers.
Keywords: 4- Nitroquinoline-1-oxide, Biomarkers, Desmoplakin, Keratin, Rat oral carcinogenesis, Squamous cell carcinoma, α6β4 integrin
How to cite this article:
Kanojia D, Sawant SS, Borges AM, Ingle AD, Vaidya MM. Alterations in keratins and associated proteins during 4- Nitroquinoline-1-oxide induced rat oral carcinogenesis. J Carcinog 2012;11:14
How to cite this URL:
Kanojia D, Sawant SS, Borges AM, Ingle AD, Vaidya MM. Alterations in keratins and associated proteins during 4- Nitroquinoline-1-oxide induced rat oral carcinogenesis. J Carcinog [serial online] 2012 [cited 2021 Oct 14];11:14. Available from: https://carcinogenesis.com/text.asp?2012/11/1/14/100861
Background
Oral squamous cell carcinoma (OSCC) is the sixth largest group of malignancies globally and represents one of the leading causes of mortality. [1] It remains a major cancer in the Indian subcontinent, comprising more than 40% of all cancer cases. The most commonly involved sites of tumor development in the Indian population are buccal mucosa and tongue. [2] Despite the advances in treatment and therapeutic modalities the five year survival rate of OSCC has not changed in the last few decades. [2] The possible reasons for poor survival rates are late detection and/or local recurrence/regional lymph node metastasis. [2]
Several studies have focused on defining tumor-specific molecular markers that can either detect cancer at an early stage or can predict patient’s outcome. [3] However, clinicopathological factors and molecular biomarkers that could identify patients at early stage or patients at high risk of recurrence/lymph node metastasis are still undefined. [4]
Keratins (K) are epithelia predominant intermediate filament proteins which are expressed in a differentiation dependent and site specific manner. [5],[6] On one hand Keratins are connected to the nuclear envelope while on the other hand, they are connected to the junctional complexes like desmosomes and hemidesmosomes through desmoplakin and plectin-β4 integrin respectively. [7] Desmosomes form cell-cell contacts while hemidesmosomes attach the cells to the basal lamina. Previous results from our laboratory have shown deregulation of paired expression of Keratins in oral cancer. [8],[9] Changes in Keratin and associated proteins like that of the desmosomes (desmoplakin) [10],[11] and hemidesmosomes (α6β4 integrin) are also documented in OSCC. [12]
To our knowledge there is no report to date about the alterations of keratins and associated proteins together during sequential stages of oral carcinogenesis. It is important to understand these molecular alterations during oral cancer development, which can help in development of early diagnostic and/or prognostic markers. In patients, the difficulty in procuring the normal tissues and tissues of all tumor stages, hampers the analysis of molecular markers. However, animal models of carcinogenesis allow the reproducible isolation of all tumor stages, including the normal tissues. [13] To this end, 4NQO induced rat model of carcinogenesis remains the preferred model for studies related to oral carcinogenesis. [13],[14]
In the present communication, we have studied the alterations in keratins and associated proteins during sequential stages of experimental oral carcinogenesis. Our results have shown alterations in keratins, desmoplakin as well as α6β4 integrin at different stages of rat oral carcinogenesis, which show a potential to be used as diagnostic/prognostic markers.
Materials and Methods
Fourty five days old male Sprague dwaley rats (weighing150-200g) were fed with standard diet and water ad libitum. They were randomly divided into three groups, 4NQO treated group (n=56), propane diol (vehicle) control group (N=56) and untreated control group (n=56) (8 animals in each of these groups were treated/untreated for 10, 20, 40,80,120,160,200 days). 12μl of 0.25% 4NQO (Sigma) was applied on each side of buccal mucosa, thrice a week for induction of tumors in buccal mucosa (60μg of 4NQO). Intramuscular atropine injections (0.04mg/Kg) were given prior to 4NQO application to prevent salivation and the animals were also kept inaccessible to drinking water after treatment for 2 hrs. 4NQO was also given in drinking water (0.001% 4NQO) for induction of tumors in tongue. Animals were weighed after every 15 days. After each time point rats were fed with normal drinking water for another 15 days. Later the animals were sacrificed using CO 2 inhalation. All experimental protocols involving animals were approved by the institutional animal ethics committee.
Keratins were isolated from lingual rat tissues using method described by Achtstaetter et al.[15] Protein estimation was carried out using RC DC Kit (Biorad). 2DE for keratins was carried out using IPG strips for first dimention (Biorad). For second dimention 3.9% stacking and 8-10% gradient separating gels were used.
SDS PAGE for desmoplakin was performed using 3.9% stacking gel and a combination of 6% top and 10% bottom gel. Western blotting was performed according to standard protocol. The primary antibodies used were AE1 (Zymed, USA, Cat No [X] 18-0153)/AE3 (Zymed, USA, Cat No [X] 18-0154), desmoplakin (Serotec, UK, Cat. No. AHP-320) and β-actin (Sigma, USA, Cat No. A 5316)
Cell lysates were prepared in SDS lysis buffer (5mM EGTA, 5mM EDTA, 0.4% SDS and protease inhibitor cocktail (Cat no. 539131) in 25mMTrisHCl pH 7.2).
Protein estimation for total cell lysates was carried out according to modified Lowry’s method. [16]
Total RNA was isolated by using Tri reagent (Sigma) from frozen tongue tissues. 2μg of RNA was reverse transcribed using MBI Fermentas cDNA synthesis kit.
For RT-PCR, reactions lacking cDNA or reverse transcriptase were run as negative controls. The following PCR primers were used: For α6, sense 5´-GTGAGGTGTGTGAACATCAG-3´ and antisense 5´-CATGGTATCGGGGAATGCT-3´ (Gene accession number – XM_001059465); for β4, sense 5´-GCAGCCCTGGGCCCAACA-3´ and antisense 5´-CCCAGGCAGGCTTTGCAG- 3´ (Gene accession number – NM_013180); for 18s sense 5´-ATGGCCGTTCTTAGTTGGTG-3´ and antisense 5´-AACGCCACTTGTCCCTCTAA-3´ (Gene accession number – V01270.1)
For immunohistochemistry paraffin embedded sections were deparaffinized, dehydrated and the antigen was retrieved using 0.1M Citrate buffer at pH 6.0 by microwave treatment. Immunohistochemistry was performed using ABC Universal Elite kit (Vector labs). The primary antibodies used were K8 (Abcam, UK, Cat.No. ab 59400), α6 integrin (Serotec, UK, Cat.No. MCA2034) and desmoplakin (Serotec, UK, Cat. No. AHP-320).
For Immunofluorescence, frozen sections were fixed with methanol and immunocytochemistry was performed using K8 (Abcam, UK, Cat.No. ab 59400) and secondary antibody (Alexafluor 488, Invitrogen).
Results
Sprague dwaley rats were sacrificed after treatment with 4NQO at different time points and dorsal tongue/buccal mucosa were dissected. [Figure 1]a1 and a5 depicts normal tongue and buccal mucosa. [Figure 1]a2 to a4 shows gross changes in the tongue and [Figure 1]a6 shows gross changes in buccal mucosa after 4NQO treatment. The tumour sizes for Papillomas/Carcinomas were 38.91 + 3.94/62.43 + 9.11mm 3 respectively [Figure 1]b. It was not possible to decipher the weights of the tumours, as the tumors have different invasive areas in the lingual tissue which makes them difficult to separate. The histopathological analysis of posterior dorsal tongue epithelium revealed no alterations in vehicle control and untreated control groups, 10, 20 and 40 days 4NQO treated lingual tissues. These tissues showed keratinizing lingual papillae [Figure 1]c1. However, 80/120 days 4NQO treated tissues exhibited dysplastic changes. The dysplastic tissues showed increase in basal cell and single cell keratinization with abnormal nuclei [Figure 1]c2. 160 days treated animals showed papillary growth of the squamous epithelium with marked parakeratosis[Figure 1]c3. At the end of 200 days all the animals demonstrated well differentiated SCC with disordered and infiltrative growth of squamous cells [Figure 1]c4[Table 1]. The analysis of buccal mucosal tissues revealed no histological alterations in vehicle treated tissue and tissues treated with 4NQO upto 160 days [Figure 1]c5. Papillomatous lesions were observed at the end of 200 days of 4NQO treatment [Figure 1]c6. The study could not be further extended beyond 200 days because the animals became moribund. For isolation of earlier stages, the experiment was repeated with higher doses of 4NQO, with 80/100μg of oral application, for 120/160 days however, the earlier stages could not be obtained.
| Figure 1: (a) Rat model of oral carcinogenesis. A. Morphology of (1) vehicle treated tongue, (2) tongue treated for 80/120 days, (3) 160 days and (4) 200 days with 4NQO. Note white patch in the posterior dorsal tongue after 80/120 days, and exophytic growth in both 160 and 200 days after 4NQO treatment as indicated by the arrow, (5) control buccal mucosa and (6) buccal mucosa treated for 200 days showing small papillomatous growth indicated by the arrow. (b)Tumor volume of papilloma and carcinomas. (c) H and E sections of (1) vehicle treated tongue, (2) tongue treated for 80/120 days (dysplasia), (3) 160 days (papillomatous lesion) and (4) 200 days (carcinoma) with 4NQO (5) Vehicle treated buccal mucosa and (6) papillomatous growth of buccal mucosa obtained after 200days of 4NQO treatment. Bar = 100ìm Click here to view |
Keratins are identified based on the molecular weight and their isoelectric point which can be separated on a 2-dimensional electrophoretic gel. Hence, in order to establish the pattern of normal rat lingual keratins we performed Mass spectrometry of the spots isolated after 2-DE of enriched Keratins. The MS analysis showed presence of K5,6a, K14 and K17 [Figure 2]a. After establishing the Keratin pattern of lingual tissues western blot was employed to understand the alterations in Keratins. Dysplastic, papillomatous lesions and carcinoma tissues demonstrated consistent up regulation of K5 and K6a as compared to normal tissue [[Figure 2] b2-4 respectively]. Further, there were no major consistent changes in other Keratins on the 2-DE gel.
| Figure 2: (a) 2DE separation and colloidal coomassie staining of enriched Keratins from normal lingual epithelium, for identification by mass spectrometry. Numbers indicate Keratin numbers. (b) Western blotting of Keratins separated on 2DE at various stages of lingual carcinogenesis. (1) Normal, (2) dysplastic, (3) pappiloma and (4) SCC tissue. Note increase in K5/6a in (2) dysplastic, (3) papillomatous lesion and (4) carcinoma tissues. Numbers indicate Keratin numbers. C. K8 expression at various stages of oral carcinogenesis using IHC. Note absence of K8 in normal tissue, however, K8 is present in suprabasal layers of (2) dysplastic tissues as indicated by arrow. Further, very high levels of K8 can be observed in (3) papillomatous lesion and (4) carcinoma tissues. Bar = 50μm. (c) The Upper Panel shows K8 Staining of normal and various stages of carcinogenesis of lingual mucosa. The middle panel shows merged image of K8 staining with nuclear stain DAPI. The lower panel shows transmission images of respective sections. Bar = 100µM Click here to view |
K8 is expressed in various malignant tissues and it plays an important role in the process of transformation. Hence, we performed immunohistochemical analysis of K8 at different stages of carcinogenesis. None of the normal tissues of any time points showed K8 expression [Figure 2]c1, however, dysplastic tissues demonstrated presence of K8 in the suprabasal layers [Figure 2] c2. Further, papillomatous lesions and carcinomas showed very high levels of K8 [Figure 2]c3 and c4. Non reactivity of antibody both in immunohistochemistry and western blotting hindered K18 analysis of rat tissues. However, it is possible that K18 is expressed in these tissues. To further substantiate our results on aberrant K8 expression we performed immunofluorescence using the same K8 antibody. As shown in the [Figure 2]c, the normal tissue does not show K8 expression whereas dysplastic, papilloma and SCC tissues showed vivid presence of K8.
Keratins on one hand are connected to the nuclear envelope while on the other hand, they are connected to the junctional complexes like desmosomes and hemidesmosomes through desmoplakin and plectin-β4 integrin respectively. It was of our interest to understand the levels and localization of Keratin associated proteins.
Hence, to study the alterations in desmoplakin levels in these tissues, Western blot analysis was performed. No changes in desmoplakin levels were observed in histologically normal tissues treated upto 40 days with 4NQO [Figure 3]b1. Dysplastic tissues showed 4.1 fold increase in desmoplakin levels [Figure 3]b2, whereas papillomatous and carcinoma tissues demonstrated 2.8 and 4.6 fold decrease in desmoplakin levels respectively [Figure 3]b3 and b4. β-actin was used as a loading control in normal, dysplasia and papillomatous tissues, whereas Cooma C blue staining was used for equal loading as the carcinoma tissues demonstrated alterations in β-actin levels [Figure 3] b4.
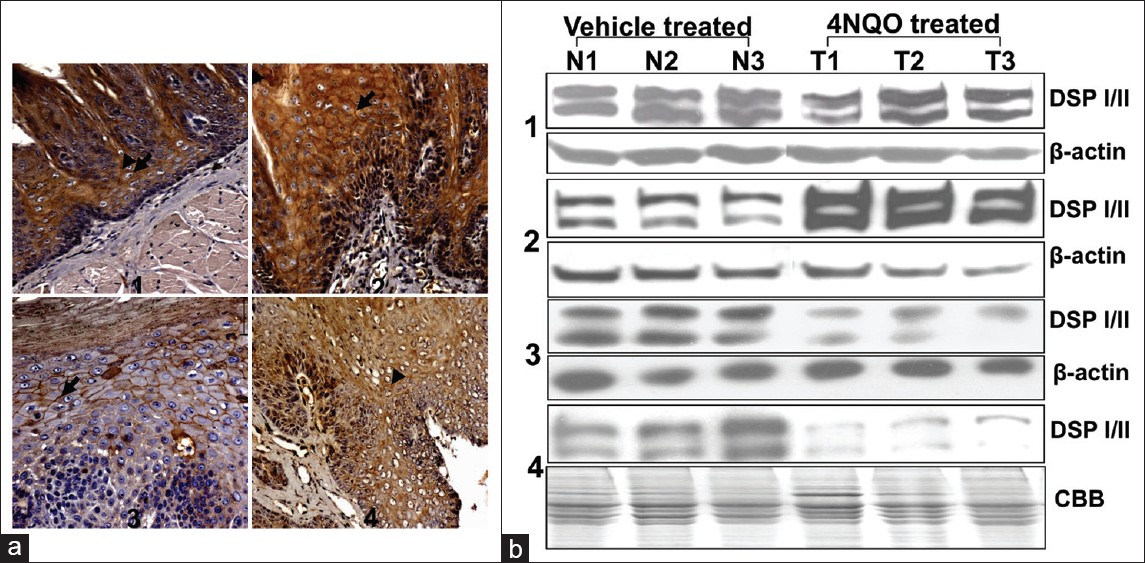 |
Figure 3: (a) Immunohistochemical analysis of desmoplakin during oral carcinogenesis. In (1) Normal tissue, desmoplakin was detected as diffused cytoplasmic and intense membranous staining in the suprabasal layers. While, in (2) dysplasia increase cytoplasmic and membranous staining of desmoplakin is evident in the suprabasal layers (arrowheads and arrow respectively). In (3) papillomatous lesion, decreased staining of desmoplakin is present in the suprabasal layers and only membranous staining can be seen (arrow). In (4) SCC decreased intensity of desmoplakin was observed. Bar = 50μm. (b) Western blot analysis for desmoplakin levels during oral carcinogenesis. N1, N2, N3 indicate vehicle treated and T1, T2, T3 indicate 4NQO treated lingual tissues. (1) Desmoplakin levels in 4NQO treated histologically normal tissues of 40 days, (2) normal and dysplastic tissues (3) normal and papillomatous tissues of 160 days (4) normal and SCC tissues of 200 days. β -actin was used as a loading control. Note increase in desmoplakin levels in (2) dysplastic tissues and decrease in (3) papillomatous lesion and (4) SCC tissues Click here to view |
Further, to analyse the alterations in desmoplakin localization at various stages of rat lingual carcinogenesis, immunohistochemical analysis was carried out. It was found that in histologically normal tissues desmoplakin was detected as diffused cytoplasmic and intense membranous staining in the suprabasal layers [Figure 3]a1. While, in dysplastic tissues increase cytoplasmic and membranous staining of desmoplakin was evident in the suprabasal layers [Figure 3] a2. In papillomatous lesion, decreased staining of desmoplakin was seen in the suprabasal layers and only membranous staining could be observed [Figure 3]a3, whereas in SCC, decreased intensity of desmoplakin was observed with diffuse cytoplasmic staining and loss of cell membrane staining [Figure 3]a4.
Inspite of repeated efforts, and using various commercially available antibodies towards rat α6β4 integrin none of the antibodies reacted in western blot. Therefore RT-PCR was carried out for both α6 and β4 integrin at different stages of lingual carcinogenesis. The dysplastic and papillomatous lesions did not show any significant change in the levels of α6 and β4 integrin mRNA [Figure 4] a1 and a2. However, SCC tissues demonstrated significant increase in α6 and β4 integrin mRNA (P<0.05, n=4) [Figure 4] a3.
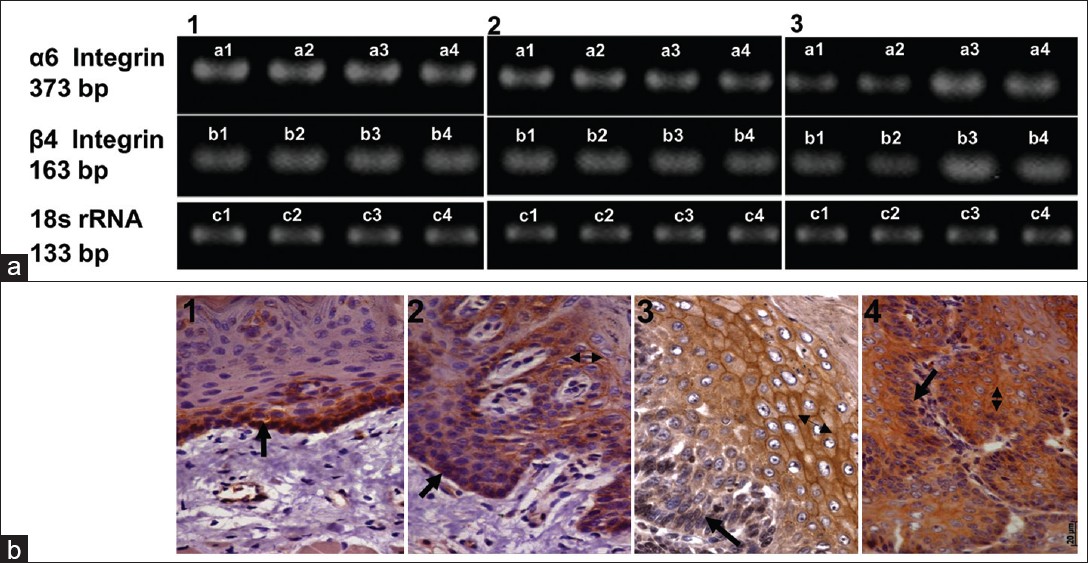 |
Figure 4: (a) RT-PCR analysis of α 6 and β 4 integrin. (1) normal and dysplastic tissues, (2) normal and papillomatous tissues, (3) normal and carcinoma tissues. Note significant increase in and RNA levels in SCC (P< 0.05 n = 4). (b) (b) Immunohistochemical analysis of α 6 integrin during oral carcinogenesis. α 6 integrin staining in (1) normal tissues, (2) dysplasia (3) papilloma and (4) SCC. Please note suprabasal expression in dysplastic and papillomatous tissues and increased staining intensity in SCC. Click here to view |
To understand the cellular localization of α6 integrin,immunohistochemical analysis was carried out. α6 integrin staining was detected only in the basal cells of vehicle treated tissues [Figure 4]b1 however, in dysplastic tissues α6 integrin was seen in the basal as well as in the suprabasal layers [Figure 4] b2. In papillomatous lesions, α6 integrin showed diffused cytoplasmic staining and intense membranous staining in suprabasal layer. Further, weak staining of integrin α6 was observed in the basal layer as compared to that seen in the control tissues [Figure 4]b3. Intense but diffused staining of α6 integrin was observed in the cytoplasm and the cell membrane of SCC [Figure 4]b4. Our RT-PCR analysis of integrin α6 showed no alterations in papillomas however, increase staining intensity of integrin α6 was observed in IHC of papilloma tissues. This difference can possibly be attributedto to the stabilization of α6 integrin protein. Since, the commercially available antibodies to β4 integrin did not react with rat tissues, we were not able to carry out immunohistochemical analysis for the same.
Taken together our analysis of Keratin expression demonstrated, increase in K5/6a levels, and ectopic K8 expression during various stages of lingual carcinogenesis. Increased desmoplakin levels were observed in dysplastic tissues (80 and 120 days) while its level decreased in papilloma and carcinoma tissues. Further, increase in suprabasal expression of α6 integrin was observed in dysplastic lesions of rat lingual epithelium which persisted in papillomas and SCC.
Discussion
Experimental animal models have proved to be an important tool, to study the molecular alterations during the process of oral carcinogenesis, because of difficulties in obtaining sequential stages of carcinogenesis in human system. In the present study, we have isolated different stages of lingual carcinogenesis.
One of the goals of our study was to establish a model for buccal mucosal carcinogenesis, which is a predominant cancer, along with SCC of tongue in the Indian subcontinent. Inspite of prevention of salivation and higher doses of 4NQO we were unable to obtain all the stages of buccal mucosal carcinogenesis. One of the possible reasons could be that the papillomatous lesion development is a rapid process and the earlier stages remained undetected. The probable reason for not obtaining SCC in buccal mucosa could be that the buccal mucosa may not have prolonged access to the carcinogen. Hence, the development of a proper model of buccal mucosal carcinogenesis still remains an area of investigation, and all the analysis was carried out only on lingual tissues.
Our analysis of Keratins expression at various stages of lingual carcinogenesis showed, increased levels of K5/K6a [Figure 2]b2-b4 and aberrant expression of K 8 in dysplastic, papillomatous lesions and carcinoma tissues of lingual epithelium [Figure 2]c2-c4. Previous reports have demonstrated suprabasal expression of K5/14 and K6/16 in premalignant and malignant lesions of oral cavity. [17] The presence of K5/14 along with CD44 has also been shown to have cancer stem cell properties in HNSCC. [18] All these reports indicate the retention of basal cell Keratins in stem cells. Thus, it is possible that the elevated levels of K5 observed in rat lingual carcinogenesis model could be indicative of retention of stem cell like properties of the premalignant and malignant cells. Previous results from our laboratory have shown down regulation of K5 expression in tobacco related oral cancer. [8],[9] These differences possibly can be explained based on the fact that although 4NQO mimics many molecular changes that occur in human oral carcinogenesis, there could be subtle differences between tumor induced by a pure carcinogen like 4NQO and the tumor induced by tobacco. Further, the keratin gene regulation is not yet well understood and the partial differences observed in Keratin expression during murine and human oral carcinogenesis, could be because of the complexity of keratin gene expression and their differential regulation.
Keratin 6 is a marker of cell proliferation and its overexpression has been observed in hyperproliferative disorders. [6] Suprabasal expression of K6/16 has been shown in oral cancer. [19] Moreover, in mouse epidermal carcinogenesis induction of K6/16 was observed in premalignant as wll as malignant skin tissues. [20] Further, cadmium and arsenite treatment to bladder epithelial cells resulted into their transformation with concomitant induction of K6a. [21] These results indicate that K6 is closely associated with proliferation of precancerous tissue and may have a role in transformation. The ectopic expression of K8 has also been shown previously by our laboratory and others. [8],[9],[22],[23] Further, our laboratory has demonstrated that forced expression of K8 in fetal buccal mucosal cells leads to cell transformation. [24] We have recently shown that downregulation of K8 results in down regulation of α6β4integrin mediated signaling and decreased cell motility and tumorogenicity. [25] These reports suggest that K8 is associated with transformation and increased malignant potential of squamous epithelial cells.
We noted increase in desmoplakin levels in dysplastic tissues [Figure 3]a2 and [Figure 3]b2 while we observed its downregulation in papillomatous lesions and SCC [Figure 3]a3, a4 and [Figure 3]b3, b4. This increase in desmoplakin in dysplastic stages may be required for efficient carcinogenesis, further, intact cell-cell adhesion through desmoplakin may be essential in supporting tumor development. Such observations have been recently made for another desmosomal protein perp. [26] However, this hypothesis needs to be confirmed in tissue specific knockout animal models of desmoplakin. Further, desmoplakin has been shown to be down regulated in a number of cancers including oral, and pharangeal cancers. [10]
Our RT-PCR analysis of integrin α6 showed no alterations in papillomas, however, increase staining intensity of integrin α6 was observed in IHC of papilloma tissues. This difference probably can be attributed to stabilization of α6 integrin protein. We have further shown over expression of α6 integrin using both RT PCR and IHC in SCC tissues. Our results of IHC have demonstrated suprabasal expression of α6 integrin in premalignant and malignant lesions. Previous reports have demonstrated that suprabasal localization of α6 integrin in premalignant and malignant tissues of human oral cavity. [27] And such altered localization is associated with poor prognosis. [28]
We have also shown overexpression of β4 integrin by RT PCR. α6β4 integrin protein overexpression has been shown in murine [29] as well as human cancers. [12] In HNSCC, overexpression of the α6β4 integrin has been associated with recurrence and poor prognosis. [12]
The conversion rate of precancer to cancer varies significantly in different studies. [30] The analysis of Keratins and associated proteins in experimental oral carcinogenesis showed that the alterations predominantly begin after the dysplastic changes in the lingual epithelium. These changes were observed in inbred animal system where carcinogen treatment results in definite conversion of premalignant lesions to carcinoma. This is indicative of the fact that the molecular changes observed can prove as useful early biomarkers if similar alterations are observed in human system.
The ongoing work in our laboratory has demonstrated ectopic expression of K8, increase desmoplakin levels and suprabasal localization of β4 integrin in human premalignant lesions (data not shown). Thus, similar results were obtained in human system and hence, some of these alterations either singly or in combination can prove useful biomarkers for early diagnosis of human oral cancer.
We have also seen alterations in Keratins, desmoplakin and α6β4 integrin in rat lingual SCC. Downregulation of desmoplakin and overexpression of α6β4 integrin separately have been shown to have prognostic value. [10],[12] Studies from our lab and others have suggested that K8/18 can also be used as prognostic markers for human oral cancer. [22] Hence, a correlation of alterations in all these proteins, with the clinical parameters can help in the development of reliable diagnostic/prognostic markers for human oral cancer. Work in this direction is already underway in our laboratory.
In summary, the 4NQO model of oral carcinogenesis, reproduces majority of the changes that are seen in human oral carcinogenesis and it can be exploited for the development of biomarkers for oral cancer.
Acknowledgment
We would like to thank Dr. G.B Maru from ACTREC for valuable suggestions in the establishment of experimental model. We acknowledge grant support from Department of Biotechnology, India. Deepak Kanojia was supported by fellowship from Council of Scientific and Industrial Research.
References
| 1. | Pisani P, BrayF, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer2002;97:72-81.  |
| 2. | Sankaranarayanan R, Black RJ, Swaminathan R, Parkin DM. An overview of cancer survival in developing countries.IARC SciPubl1998;145:135-73.  [PUBMED] |
| 3. | Lippman SM, Hong WK. Molecular markers of the risk of oral cancer. N Engl J Med2001;344:1323-6.  [PUBMED] |
| 4. | Schliephake H. Prognostic relevance of molecular markers of oral cancer–a review. Int J Oral MaxillofacSurg2003;32:233-45.  [PUBMED] |
| 5. | MollR, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell1982;31:11-24.  |
| 6. | Vaidya MM, Kanojia D. Keratins: Markers of cell differentiation or regulators of cell differentiation?J Biosci2007;32:629-34.  [PUBMED] |
| 7. | Luna EJ, Hitt AL. Cytoskeleton–plasma membrane interactions. Science1992;258:955-64.  [PUBMED] |
| 8. | Vaidya MM, Borges AM, Pradhan SA, Bhisey AN. Cytokeratin expression in squamous cell carcinomas of the tongue and alveolar mucosa. Eur J Cancer B Oral Oncol1996;32B:333-6.  [PUBMED] |
| 9. | Vaidya MM, Sawant SS, Borges AM, Ogale SB, Bhisey AN. Cytokeratin expression in precancerous lesions of the human oral cavity. Oral Oncol1998;34:261-4.  [PUBMED] |
| 10. | Papagerakis S, Shabana AH, Pollock BH, Papagerakis P, Depondt J, Berdal A. Altered desmoplakin expression at transcriptional and protein levels provides prognostic information in human oropharyngeal cancer. Hum Pathol2009;40:1320-9.  [PUBMED] |
| 11. | Depondt J, Shabana AH, Florescu-Zorila S, Gehanno P, Forest N. Downregulation of desmosomal molecules in oral and pharyngeal squamous cell carcinomas as a marker for tumor growth and distant metastasis. Eur J Oral Sci1999;107:183-93.  [PUBMED] |
| 12. | Wolf GT, Carey TE, Schmaltz SP, McClatchey KD, Poore J, Glaser L, et al.Altered antigen expression predicts outcome in squamous cell carcinoma of the head and neck. J Natl Cancer Inst1990;82:1566-72.  |
| 13. | Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol2006;42:655-67.  [PUBMED] |
| 14. | Vered M, Yarom N, Dayan D. 4NQO oral carcinogenesis: Animal models, molecular markers and future expectations. Oral Oncol2005;41:337-9.  |
| 15. | Achtstaetter T, Hatzfeld M, Quinlan RA, Parmelee DC, Franke WW.Separation of cytokeratin polypeptides by gel electrophoretic and chromatographic techniques and their identification by immunoblotting.Methods Enzymol1986;134:355-71.  [PUBMED] |
| 16. | Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem1977;83:346-56.  [PUBMED] |
| 17. | Kannan S, Balaram P, Chandran GJ, Pillai MR, Mathew B, Nalinakumari KR, et al. Alterations in expression of terminal differentiation markers of keratinocytes during oral carcinogenesis. Pathobiology1994;62:127-33.  |
| 18. | Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. ProcNatlAcadSci U S A2007;104:973-8.  |
| 19. | Heyden A, Huitfeldt HS, Koppang HS, Thrane PS, Bryne M, Brandtzaeg P. Cytokeratins as epithelial differentiation markers in premalignant and malignant oral lesions. J Oral Pathol Med1992;21:7-11.  |
| 20. | Santos M, BallestinC, Garcia-MartinR, JorcanoJL.Delays in malignant tumor development in transgenic mice by forced epidermal keratin 10 expression in mouse skin carcinomas. MolCarcinog1997;20:3-9.  |
| 21. | Somji S, Bathula CS, Zhou XD, Sens MA, Sens DA, Garrett SH: Transformation of human urothelial cells (UROtsa) by as and cd induces the expression of keratin 6a. Environ Health Perspect2008;116:434-40.  |
| 22. | Fillies T, Werkmeister R, Packeisen J, Brandt B, Morin P, Weingart D, et al.Cytokeratin 8/18 expression indicates a poor prognosis in squamous cell carcinomas of the oral cavity. BMC Cancer2006;6:10.  |
| 23. | Ogden GR, Chisholm DM, Adi M, Lane EB. Cytokeratin expression in oral cancer and its relationship to tumor differentiation.J Oral Pathol Med1993;22:82-6.  |
| 24. | Raul U, Sawant S, Dange P, Kalraiya R, Ingle A, Vaidya M. Implications of cytokeratin 8/18 filament formation in stratified epithelial cells: Induction of transformed phenotype. Int J Cancer2004;111:662-8.  |
| 25. | Alam H, Kundu ST, Dalal SN, Vaidya MM. Loss of keratins 8 and 18 leads to alterations in alpha6beta4-integrin-mediated signalling and decreased neoplastic progression in an oral-tumour-derived cell line. J Cell Sci2011;124(Pt 12):2096-106.  |
| 26. | Marques MR, Horner JS, Ihrie RA, Bronson RT, Attardi LD. Mice lacking the p53/p63 target gene Perp are resistant to papilloma development. Cancer Res2005;65:6551-6.  |
| 27. | Garzino-Demo P, Carrozzo M, Trusolino L, Savoia P, Gandolfo S, Marchisio PC. Altered expression of alpha 6 integrin subunit in oral squamous cell carcinoma and oral potentially malignant lesions.Oral Oncol1998;34:204-10.  |
| 28. | Van Waes C. Cell adhesion and regulatory molecules involved in tumor formation, hemostasis, and wound healing. Head Neck1995;17:140-7.  |
| 29. | Tennenbaum T, Yuspa SH, Grover A, Castronovo V, Sobel ME, YamadaY, et al.Extracellular matrix receptors and mouse skin carcinogenesis: Altered expression linked to appearance of early markers of tumor progression. Cancer Res1992;52:2966-2976  |
| 30. | Neville BW, Day TA. Oral cancer and precancerous lesions.CA Cancer J Clin2002;52:195-215.  |
Authors
Dr. Deepak Kanojia, Department of Biological Science, University of South Carolina, Columbia, SC 29208
Dr. Anita M Borges, Department of Histopathology, Asian Institute of Oncology, S.L. Raheja Hospital, Mahim, Mumbai – 400 016, India.
Dr. Arvind D Ingle, Laboratory animal facility, Advanced Centre for Treatment, Research and Education in CancerTata Memorial Centre Sector 22, Kharghar,Navi Mumbai – 410 210, India.
Mrs. Sharada S Sawant, Vaidya Lab, Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre, Kharghar, Navi Mumbai- 410210, Maharashtra India
Dr. Milind M Vaidya, Vaidya Lab, Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre, Kharghar, Navi Mumbai- 410210, Maharashtra India
Figures
[Figure 1], [Figure 2], [Figure 3], [Figure 4]
Tables
