Jason A Zell1, Bruce S Lin2, Argyrios Ziogas3, Hoda Anton-Culver3
1 Department of Epidemiology; Genetic Epidemiology Research Institute; Chao Family Comprehensive Cancer Center and Department of Medicine, Division of Hematology/Oncology, University of California, Irvine, California, USA
2 Chao Family Comprehensive Cancer Center; Department of Medicine, Division of Hematology/Oncology, University of California, Irvine, California, USA
3 Department of Epidemiology; Genetic Epidemiology Research Institute, University of California, Irvine, California, USA
| Date of Submission | 22-May-2012 |
| Date of Acceptance | 16-Sep-2012 |
| Date of Web Publication | 28-Nov-2012 |
Correspondence Address:
Jason A Zell
Department of Epidemiology; Genetic Epidemiology Research Institute; Chao Family Comprehensive Cancer Center and Department of Medicine, Division of Hematology/Oncology, University of California, Irvine, California
USA
Source of Support: This work was supported by grants from the National Institutes of Health (USA), CA78134 (H. Anton-Culver), CA78285 (H. Anton-Culver), K23 CA133142 (J.A. Zell), and L30 CA130160 (J.A. Zell). BS Lin was supported in part by a 2010- 2011 Oncology Clinical Fellowship Award from Pfizer’s Medical and Academic Partnership Program., Conflict of Interest: None
DOI: 10.4103/1477-3163.104004
Abstract
Background: Dietary arginine and meat consumption are implicated in colorectal cancer (CRC) progression via polyamine-dependent processes. Polymorphism in the polyamine-regulatory gene, ornithine decarboxylase 1 (Odc1, rs2302615) is prognostic for CRC-specific mortality. Here, we examined joint effects of meat consumption and Odc1 polymorphism on CRC-specific mortality. Materials and Methods: The analytic cohort was comprised of 329 incident stage I-III CRC cases diagnosed 1994-1996 with follow- up through March 2008. Odc1 genotyping was conducted using primers that amplify a 172-bp fragment containing the polymorphic base at +316. Dietary questionnaires were administered at cohort entry. Multivariate Cox proportional hazards regression analysis for CRC-specific mortality was stratified by tumor, node, metastasis (TNM) stage, and adjusted for clinically relevant variables, plus meat consumption (as a continuous variable, i.e., the number of medium-sized servings/week), Odc1 genotype, and a term representing the meat consumption and Odc1 genotype interaction. The primary outcome was the interaction of Odc1 and meat intake on CRC-specific mortality, as assessed by departures from multiplicative joint effects. Results: Odc1 genotype distribution was 51% GG, 49% GA/AA. In the multivariate model, there was a significant interaction between meat consumption and Odc1 genotype, P-int = 0.01. Among Odc1 GA/AA CRC cases in meat consumption Quartiles 1-3, increased mortality risk was observed when compared to GG cases (adjusted hazards ratio (HR) = 7.06 [95% CI 2.34-21.28]) – a difference not found among cases in the highest dietary meat consumption Quartile 4. Conclusions: Effects of meat consumption on CRC-specific mortality risk differ based on genetic polymorphism at Odc1. These results provide further evidence that polyamine metabolism and its modulation by dietary factors such as meat may have relevance to CRC outcomes.
Keywords: Colorectal cancer, meat consumption, mortality, ornithine decarboxylase, ornithine decarboxylase l, polyamines, single nucleotide polymorphism
How to cite this article:
Zell JA, Lin BS, Ziogas A, Anton-Culver H. Meat consumption, ornithine decarboxylase gene polymorphism, and outcomes after colorectal cancer diagnosis. J Carcinog 2012;11:17
How to cite this URL:
Zell JA, Lin BS, Ziogas A, Anton-Culver H. Meat consumption, ornithine decarboxylase gene polymorphism, and outcomes after colorectal cancer diagnosis. J Carcinog [serial online] 2012 [cited 2021 Oct 13];11:17. Available from: https://carcinogenesis.com/text.asp?2012/11/1/17/104004
Background
Abnormalities in the control of polyamine metabolism and transport result in increased polyamine levels that can promote tumorigenesis in the colorectum and other tissues.[1] For instance, polyamine metabolism is upregulated in intestinal epithelial tissues of humans with familial adenomatous polyposis (FAP)[2] a syndrome associated with high risk of colon and other cancers. FAP is caused by mutations in the adenomatous polyposis coli (APC) tumor suppressor gene, and wild-type APC signaling downregulates ornithine decarboxylase 1 (Odc1, i.e., the gene encoding ornithine decarboxylase (ODC), which is the rate-limiting enzyme in polyamine biosynthesis) 0expression in both human cells[3] and in a FAP mouse model.[4] The relevance of polyamine inhibition as a target for therapeutic prevention of colorectal neoplasia in humans has been demonstrated with the selective ODC inhibitor D, L-α-Difluoromethylornithine (DFMO, eflornithine) in combination with sulindac (a nonsteroidal anti-inflammatory drug [NSAID] that promotes cellular polyamine export[5] via induction of spermidine spermine acetyltransferase [SSAT]). In a randomized controlled clinical trial of individuals with history of colorectal adenomas, the combination of eflornithine and sulindac compared to placebo markedly lowered the adenoma recurrence rate.[6] The observed clinical effects of this therapeutic prevention regimen appear to be mediated by polyamine-inhibitory mechanisms and not due to the cyclooxygenase-inhibitory mechanisms of sulindac.[7] The robust effects of these polyamine-inhibitory agents against colorectal adenomas, and in particular high-risk adenomas (multiple adenomas, advanced adenomas) were achieved at the cost of moderate subclinical ototoxicity[8] that may be related to genetic polymorphism in Odc1,[9] with no clear differences in gastrointestinal or cardiovascular toxicity.[6],[10] These recent clinical findings, demonstrating efficacy against recurrent colorectal adenomas with a favorable safety profile, have spawned new interest in polyamine inhibition of colorectal carcinogenesis.
Gene-environment interactions clearly play a role in colorectal cancer (CRC) risk, and it is believed that such interactions influence CRC progression. However, relatively little is known about gene-environment effects on outcomes after CRC diagnosis. Our group has previously demonstrated that the Odc1 A-allele is an adverse prognostic factor after diagnosis of stage I-III (i.e., nonmetastatic) CRC.[11] Meat consumption is a major polyamine-related exposure commonly encountered in the CRC survivorship population, as meat is high in arginine content – the direct precursor to ornithine which itself undergoes conversion by ODC to form the various polyamines: Putrescine, spermidine, and spermine.[12] In ApcMin/+ mice, dietary arginine is associated with increased tissue polyamine levels and increased incidence of high-grade intestinal adenomas.[13] Among human CRC cases, total daily arginine content is dependent on meat consumption, and familial CRC cases consuming the highest quartile of meat intake experience adverse survival outcomes compared to those in lower meat intake quartiles.[13]
Supporting evidence for the adverse effects of diet on outcomes after CRC diagnosis comes from a separate report of stage III (lymph node positive) colon cancer patients, where high consumption of a Western dietary pattern (i.e., a diet high in meat, fat, refined grains, and dessert) was associated with decreased time to recurrence and decreased overall survival.[14] Additionally, an observed CRC-specific mortality risk reduction ascribed to NSAID use prior to diagnosis[15] was found to be restricted to CRC cases reporting low levels of meat consumption prior to diagnosis, consistent with a polyamine-inhibitory process.[16] Taken together, these results suggest potential roles for polyamine-related genetic alterations (Odc1 polymorphism) and environmental exposures (dietary meat consumption) on clinical outcomes after CRC diagnosis. A large body of literature associates meat consumption (and particularly processed meat consumption) with increased risk of CRC in humans.[17] However, despite the possible relationship of meat consumption to polyamine regulation, no prior studies have investigated the joint effects of polyamine-related dietary and genetic factors among CRC cases. We have designed the present study to evaluate the joint effects of dietary meat consumption and genetic polymorphism at Odc1 with CRC-specific mortality among nonmetastatic CRC cases.
Methods
Study population
We studied incident cases of invasive CRC with stage I-III disease at presentation enrolled in the University of California, Irvine Gene-Environment Study of Familial CRC[13],[18] during 1994-1996 with follow-up through March 2008. Patients with advanced (stage IV) CRC at presentation were excluded as they have a much higher event rate (rate of death), and the majority of such patients are treated indefinitely with palliative chemotherapy. Participants were identified through the population-based cancer registries of the Cancer Surveillance Program of Orange County/San Diego Imperial Organization for Cancer Control as previously described.[18] At study entry, cases signed a consent form allowing for blood draws and medical record release. The study was approved by the University of California (UC) Irvine Institutional Review Board (#93-257). Clinical and demographic data including vital status and follow-up were obtained through linkage to the regional cancer registry databases as previously described.[13],[18],[19] Tumor grade and tumor, node, metastasis (TNM) staging determination were derived from existing American Joint Commission on Cancer (AJCC) codes where available. Otherwise stage was derived via conversion of extent of disease codes, as previously reported.[20] Family history of cancer in a first-degree relative was ascertained through telephone interview-based self-reporting at time of enrollment.[19],[21] Twenty-two cases with hereditary nonpolyposis colon cancer (HNPCC), as defined by Amsterdam criteria, were identified and excluded from the analysis. The median time from CRC diagnosis until study entry (i.e., date of family history interview) was 18 months (95% CI 12-32 months).
DNA extraction and Odc1 + 316 single nucleotide polymorphism (SNP) genotyping
DNA was extracted from 2.0 mL red blood cell clot samples using the QIAGEN QIAamp DNA Midi or Mini Kits (Qiagen), as previously described. [11] Genotyping of the Odc1 + 316 single nucleotide polymorphism (SNP) (National Center for Biotechnology Information SNP database ID rs2302615) was conducted with oligonucleotide primers designed to amplify a 172-bp fragment containing the polymorphic base at position +316 (Applied Biosystems, Foster City, CA). Allele-specific TaqMan probes were synthesized with different 5′ labels (6-carboxyfluorescein or VIC) and the same 3′ quencher dye (6-carboxytetramethylrhodamine). [11],[22] Each PCR reaction (5 μL total) contained 10 ng of participant DNA, 30 pmol of each primer, 12.5 pmol of each TaqMan probe, and 1x TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), as previously reported. [11],[22],[23] Out of 481, 440 DNA samples were successfully genotyped. Forty-one cases (8.5%) resulted in an undetermined Odc1 + 316 genotype due to low DNA concentration and/or poor DNA quality; however, no significant clinicopathological differences were observed between the successfully genotyped and unsuccessfully genotyped cases as previously reported. [11] Forty cases had stage IV CRC at diagnosis and were excluded. Of the remaining 400 stage I-III CRC cases, 335 completed the dietary questionnaire including 329 who completed questions related to meat consumption (representing 82% of 400 eligible stage I-III CRC cases). No significant differences were observed between the group completing the food frequency questions (FFQ) related to meat consumption (n = 329) and the group not completing the FFQ (n = 71) based on age, gender, race/ethnicity, TNM stage, tumor subsite location, histology, tumor grade, family history of CRC, treatment (surgery radiation, chemotherapy), or Odc1 genotype. When analyzed by three genotypes (GG, GA, and AA), Odc1 genotype distribution by ethnicity revealed no significant differences: Caucasian (290 cases: 52% GG, 41% GA, 7% AA, minor-A allele frequency = 27%), African-American (3 cases: 67% GG, 33% GA, 0% AA, minor-A allele frequency = 17%), Hispanics (16 cases: 50% GG, 50% GA, 0% AA, minor-A allele frequency = 25%), and Asians (20 cases: 30% GG, 50% GA, 20% AA, minor-A allele frequency = 45%) (P = 0.18). Within each race, Odc1 genotype distribution was in Hardy-Weinberg equilibrium (Caucasians, P = 0.45; African-Americans, P = 0.73; Hispanics, P = 0.18; and Asians, P = 0.96).
Assessment of dietary intake
Food consumption was self-reported via a validated 100-item National Cancer Institute Block food frequency questionnaire (FFQ) administered at baseline (cohort entry), where patients were asked to report their usual eating habits for 1 year before CRC diagnosis. [23] Micronutrient data, total daily fiber intake, and total daily energy intake were calculated from the self-reported FFQ responses. [24] The types of meat queried for this analysis were beef roast or beef steaks or beef sandwiches, beef stew or pot pie, burrito or taco with meat, hamburger or cheeseburger, hot dogs, liver (including chicken livers), lunch meat (including ham, bologna, other lunch meats made with or without turkey), other meat soups, pork (including pork chops and pork roast), sausage, chicken or turkey, chicken stew or mixed chicken dish, fried chicken, fried fish, other types of fish, oysters, shellfish, and tuna. Consumption of each meat item was converted to the number of medium-sized servings per week by multiplying the frequency of servings per week (never, once a month, 2-3 times/month, once a week, 2 times/week, 3-4 times/ week, 5-6 times/week, everyday) by the estimated serving size (0.5 for small, 1.0 for medium, and 1.5 for large) as previously described. [13],[16] CRC patients were divided into quartiles based on their meat consumption of medium-sized servings: Quartile 1, 0.00-4.72 servings/week; quartile 2, 4.73-7.26 servings/week; quartile 3, 7.27-11.00 servings/week; and quartile 4, ≥11.01 servings/week. Cases in the highest quartile of meat consumption (Q4) were compared to those in all of the other meat consumption quartiles (Q1-Q3). Manual chart review of the dietary FFQ was conducted on all cases reporting total daily energy intake of less than 500 kcal or greater than 5,000 kcal, [13] and after review all data were considered valid and included in the final analysis.
Statistical analysis
Comparisons of demographic, clinical, and pathological variables among CRC cases were done using Pearson Chi-square statistic or Fisher’s exact test for nominal variables and Kruskal-Wallis tests were used for two-group comparisons of nonparametric data. An estimated 1:1 ratio of Odc1 GG genotype to Odc1 GA/AA genotype was expected based on previous literature.[22],[23],[24],[25] CRC-specific mortality was defined as death due to CRC, and data were censored in the following instances: Alive at the end of follow-up, loss to follow-up, or death from any cause other than CRC. Cox proportional hazards modeling was performed for all CRC cases using time since diagnosis to profile the adjusted risk of CRC-specific death based on Odc1 genotype. Multivariable analyses assessing the interaction between Odc1 genotype and meat consumption on CRC-specific mortality were done with TNM stage at diagnosis fit as a stratum variable in the Cox proportional hazards model and adjustment for age, gender, ethnicity, family history of CRC, tumor site within the colon, histological subtype, treatment with surgery, radiation therapy, chemotherapy, and meat consumption (as a continuous variable, i.e., the number of medium-sized meat servings per week), with or without the interaction term (as assessed by departures from multiplicative joint effects: Meat intake × Odc1 genotype). Similarly, Cox regression analyses assessing the effects of Odc1 genotype on mortality were also performed separately for each case of meat consumption quartile, and then in collapsed categorical groups based on the similar estimates for Q1-Q3 versus Q4. For covariates with categorical data, dummy coding was assigned (0 or 1) and each category was included in the multivariate models in comparison to the referent group. Odc1 genotype was analyzed using the dominant genetic model (GG vs. GA/ AA) using dummy variables with GA/AA coded as 1 and GG as the referent group. The dominant genetic model was selected as risk estimates for individual genotypes in the full additive model (GG, GA, and AA) revealed that compared to Odc1 GG as a referent group, GA and AA risk estimates were within 15% of each other. All analyses were conducted using SAS 9.2 statistical software (SAS Institute, Cary, NC).
Results
Three hundred and twenty-nine stage I-III CRC cases with available meat consumption data identified from the UC Irvine CRC gene-environment study were used in the case-only analysis. Median follow-up duration was 11 years, 1 month. There were 209 (64%) colon cancer cases, 116 (35%) rectal cancer cases, and 4 (1%) CRC cases of unspecified location. Clinicopathological data for CRC cases are shown in [Table 1]. Timing of radiation therapy was available for 51 cases (46 rectal and 5 colon cancer cases) receiving radiation therapy. Twelve (23.5%) received neoadjuvant (i.e., presurgery) radiation, 37 (72.6%) received adjuvant (i.e., postoperative) radiation, and 2 (3.9%) received combination of neoadjuvant and adjuvant radiation therapy. Odc1 genotype distribution among all CRC cases was 167 (51%) GG, 139 (42%) GA, and 23 (7%) AA. There were no significant differences in Odc1 genotype distribution (GG vs. GA/AA) by age, median meat consumption, gender, ethnicity, family history, stage, site within the colorectum, histology, tumor grade, surgical treatment, radiation therapy, or chemotherapy [Table 1]. There were no significant differences among the clinicopathological characteristics between Odc1 GG versus GA/AA groups when separated by meat consumption score quartiles except for ethnicity in quartile 3 [Table 2].
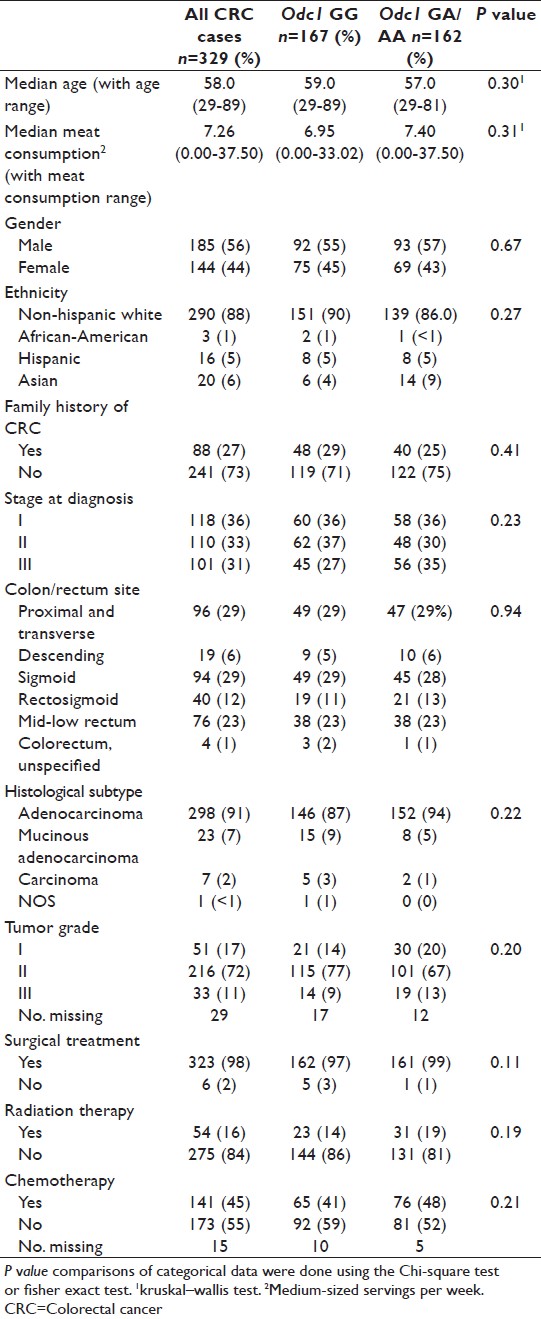 |
Table 1: Descriptive analysis for colorectal cancer cases overall and based on Odc1 genotype Click here to view |
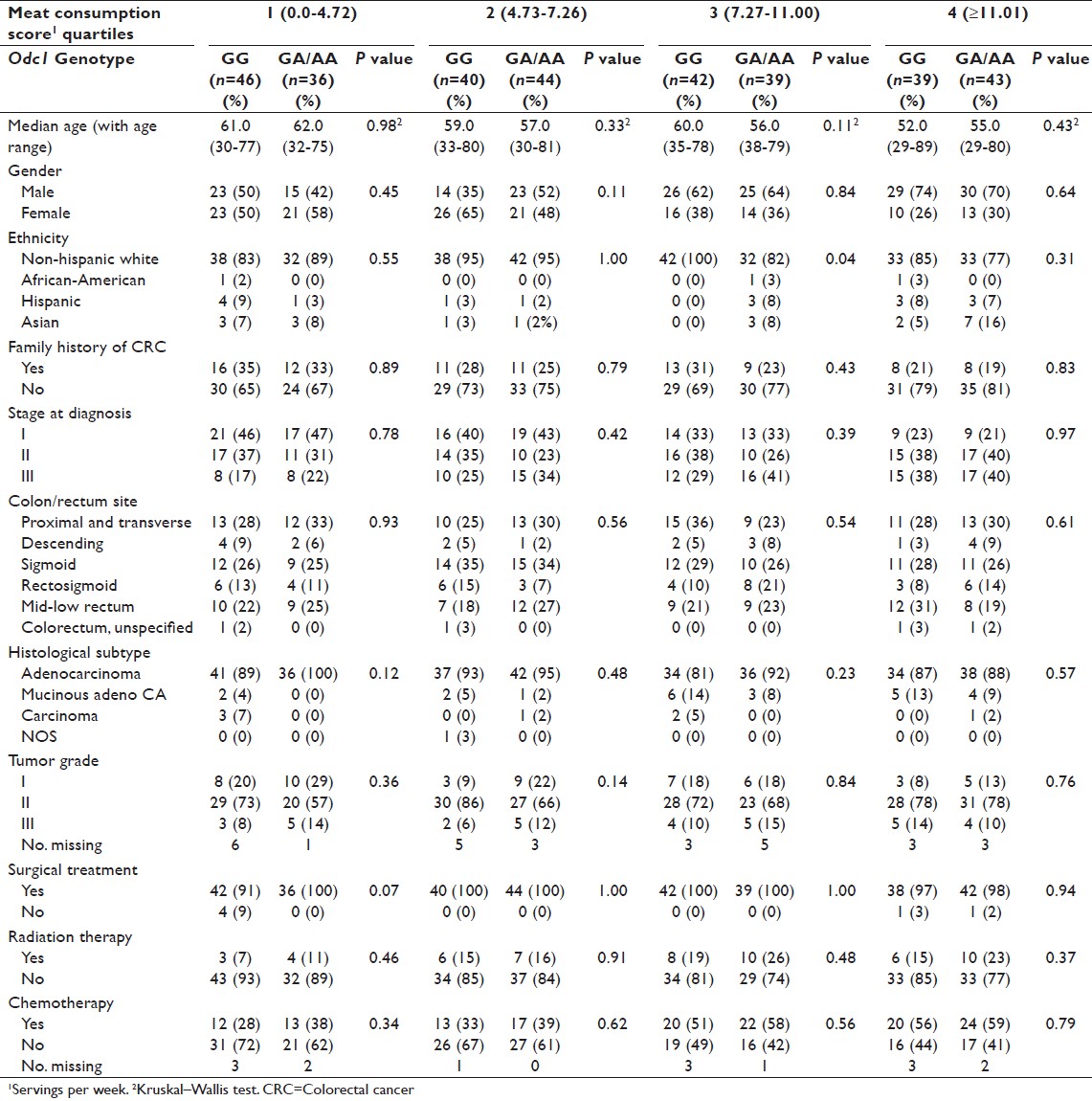 |
Table 2: Descriptive analysis for colorectal cancer cases overall and based on Odc1 genotype and meat consumption score quartiles Click here to view |
Of the 329 stage I-III CRC cases, 92 (28%) were deceased at the time of analysis. Thirty-six (39%) deaths occurred in cases carrying the Odc1 GG genotype, compared to 56 (61%) deaths in cases with the GA/AA genotypes. Cause of death was available for 63 of the 92 deceased CRC cases. Forty-eight (76%) CRC cases died as a result of CRC. A statistically significant decrease in CRC-specific mortality was observed among CRC cases homozygous for the Odc1 G-allele (10-year morality = 9%) compared to cases with at least one A-allele (Odc1 GA/AA) (10-year mortality = 20%; P = 0.0025), as previously reported.[11]
CRC-specific mortality estimates based on Odc1 genotype in the full regression model were as follows: Compared to the Odc1 G-allele as a referent, the adjusted hazards ratio (HR) for the Odc1 A-allele was 12.75, P = 0.001. In unadjusted analyses, the Odc1 A-allele HR was 2.29, P = 0.008 [Table 3]. As a main effect, meat consumption was associated with increased CRC-specific mortality in the adjusted analyses (HR = 1.16, 95% CI 1.04-1.30, P = 0.006). The interaction between Odc1 A-allele and meat consumption in the adjusted model was significant with a P value of 0.01. Multivariate overall mortality estimates showed a similar trend with Odc1 G-allele conferring a prognostic advantage, Odc1 G-allele HR = 1.00 (referent), Odc1 A-allele adjusted HR = 2.55, P = 0.033 (unadjusted Odc1 A-allele HR = 1.74, P = 0.0098).
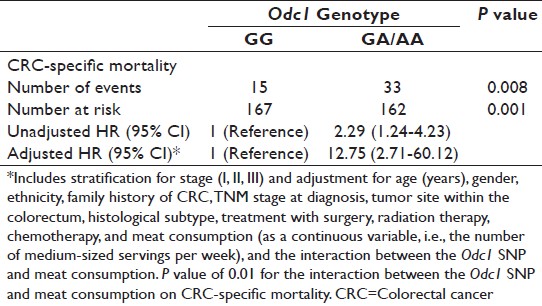 |
Table 3: Multivariate colorectal cancer–specific mortality analysis for colorectal cancer cases based on ornithine decarboxylase 1 genotype Click here to view |
Subset multivariate CRC-specific mortality analysis revealed that the Odc1 A-allele ( vs. Odc1 GG as a referent group) confers a higher risk of CRC-specific mortality in the meat consumption quartiles 1-3, but not in the highest meat consumption quartile: Q1 HR = 11.3 (P = 0.06), Q2 HR = 9.2 (P = 0.09), Q3 HR = 8.8 (P = 0.05), and Q4 HR = 0.52 (P = 0.29). As risk estimates were similar in Q1-3, the risk estimates for the collapsed categorical group (Q1-Q3) versus Q4 are presented in [Table 4]. In the collapsed categorical Q1-Q3 group, using Odc1 GG as a referent, the Odc1 A-allele HR is 7.06, P = 0.0005 (unadjusted Odc1 A-allele HR = 5.85, P = 0.0003) [Table 4]. In contrast, among cases in meat consumption Q4, no significant differences were detected for CRC-specific mortality risk based on Odc1 genotype (compared with Odc1 GG cases as a referent, the adjusted HR for GA/AA cases was 0.52, 95% CI 0.16-1.73, P = 0.29). On univariate analysis, lower CRC-specific mortality was observed among CRC cases homozygous for the Odc1 G-allele in the group reporting meat consumption in first three quartiles (Q1-Q3), Odc1 G-allele 10-year mortality = 4% versus A-allele 10-year mortality = 22%, P = <0.0001 [Figure 1]. In the highest meat consumption group (Q4), CRC-specific mortality differences for CRC cases based on Odc1 genotype were not statistically different (Odc1 A-allele 10-year mortality = 17% versus G-allele 10-year mortality = 27%; P = 0.31) [Figure 2].
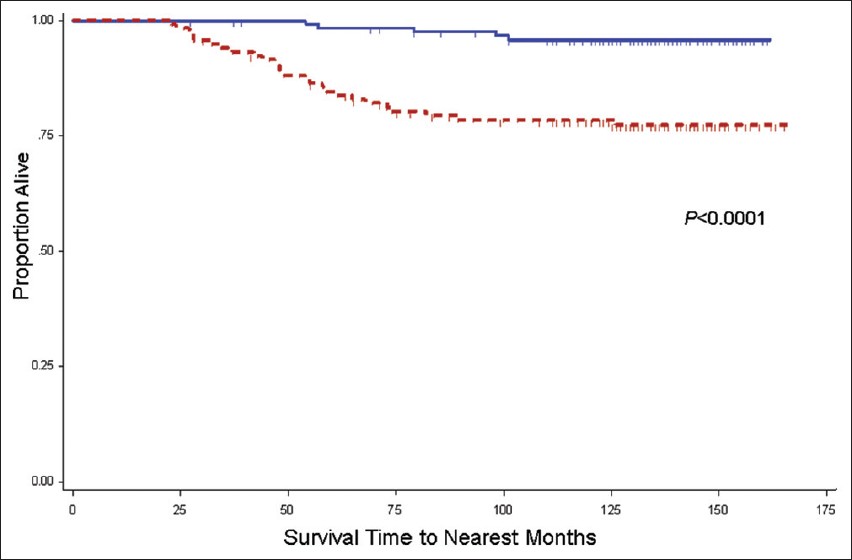 |
Figure 1: Kaplan-Meier colorectal cancer (CRC)-specific mortality rate estimates for stage I-III CRC cases in meat consumption quartiles 1-3, stratified by ornithine decarboxylase 1 (Odc1) +316 genotype. Includes cases from the University of California Irvine Gene-Environment Study of Familial CRC diagnosed during 1994-1996 with follow-up through March 2008; Solid line, Odc1 GG (128 cases, 5 CRC-specific deaths); Dashed line, Odc1 GA/AA (119 cases, 26 CRC-specific deaths). Vertical tic marks represent censored observations Click here to view |
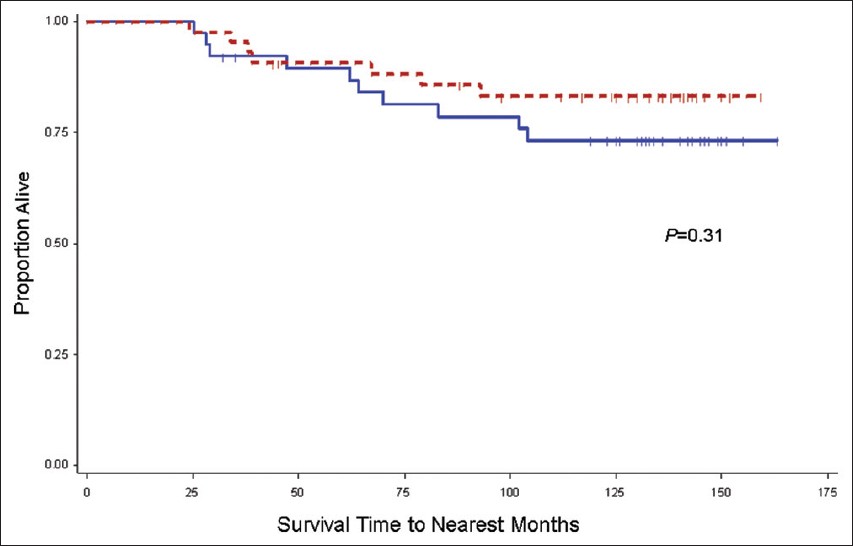 |
Figure 2: Kaplan-Meier colorectal cancer (CRC)-specif ic mortality rate estimates for stage I-III CRC cases in meat consumption quartile 4, stratified by ornithine decarboxylase 1 (Odc1) +316 genotype. Includes cases from the University of California Irvine Gene-Environment Study of Familial CRC diagnosed during 1994-1996 with follow-up through March 2008; Solid line, Odc1 GG (39 cases, 10 CRC-specific deaths); Dashed line, Odc1 GA/AA (43 cases, 7 CRC-specific deaths). Vertical tic marks represent censored observations Click here to view |
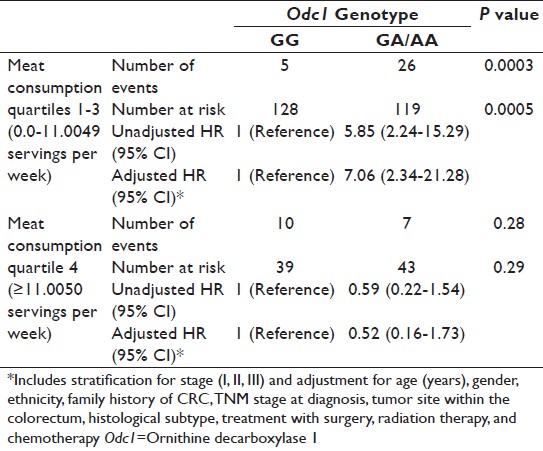 |
Table 4: Multivariate colorectal cancer–specific mortality analysis for CRC cases based on ornithine decarboxylase 1 genotype and meat consumption group Click here to view |
Discussion
In this population-based analysis of CRC cases, we observed a significant interaction between meat consumption and +316 Odc1 genotype with regard to CRC-specific mortality. A statistically significant increased risk of CRC-specific mortality was observed for Odc1 GA/AA CRC cases in meat consumption quartiles 1-3 compared to Odc1 GG cases, a finding which was observed after stratification for stage and adjustment for age, gender, and clinically relevant factors. Of note, increased meat consumption itself was independently associated with increased CRC-specific mortality in this study (P = 0.006). The +316 Odc1 SNP was chosen since it is the only SNP in the polyamine pathway that has been associated with differential survival outcomes in CRC cases.[11] The results presented here support the hypothesis that polyamine metabolism and its modulation by specific dietary factors have relevance on CRC outcomes.
Dietary influences relevant to polyamine regulation in humans with colorectal neoplasia have been investigated in a secondary analysis of data from the randomized, placebo-controlled phase III trial of DFMO, eflornithine + sulindac versus placebo among colorectal adenoma patients.[6] In the parent trial, a 70% reduction in recurrent colorectal adenomas was observed in the combination of eflornithine and sulindac group compared with the placebo group. Using dietary information recorded in the trial, a polyamine database was developed.[26] Major contributors of dietary polyamines include meat (ground meat, lunch meat, lasagna/pasta with meat sauce) as well as other sources such as green peas, peanut butter, peanuts, other nuts, corn, grapefruit juice, orange juice, and beer.[26] Interestingly, a greater proportion of patients consuming the highest quartile of dietary polyamine had large adenomas at baseline compared to patients in the remaining three dietary polyamine quartiles.[27] Furthermore, the preventive effect of the pharmacological polyamine-inhibitory intervention was abrogated in patients consuming high quartiles of dietary polyamines at baseline. A statistically significant interaction was observed between dietary polyamine group and treatment with regard to colorectal adenoma recurrence – a finding that remained after adjustment for genetic polymorphism at Odc1. These findings, when considered together with the findings from the present manuscript, suggest key roles for polyamine-related dietary influences on CRC progression. Ultimately, confirmation of the findings presented in this manuscript may emerge from future studies, such as the phase III post-adjuvant randomized double-blind trial of eflornithine, sulindac, placebo alone, or in combination among stage 0, I, II, or III colon cancer patients (which has pharmacogenetic and dietary substudies embedded within the protocol).[28]
Other studies of genetic variation in Odc1 on CRC progression must be considered in light of the results presented here. In colorectal adenoma patients, the Odc1 + 316 SNP may be prognostic for colorectal adenoma recurrence,[23] especially in association with aspirin usage.[23],[24],[25] Recently, the relevance of the rs2302615 Odc1 SNP to colorectal adenoma recurrence has been challenged, as associations were detected for other downstream SNPs in Odc1, but not in rs2302615.[29] Clearly, further research must be done to sort out the contributions of genetic polymorphism in Odc1 on the tumorigenic process. Little is known about how genetic polymorphism at Odc1 influences outcomes in CRC. One study demonstrated no association with Odc1 polymorphism on CRC risk,[30] despite the aforementioned associations of Odc1 with mortality outcomes after CRC diagnosis.[11]
This observational study shares limitations of other population-based analyses, including lack of data on comorbid conditions, performance status, or particular chemotherapeutic regimens utilized. There is also a potential for selection bias, favoring a relatively healthy group of CRC survivors, since there was a median 18-month delay from the time of CRC diagnosis until study enrollment. The sample size is relatively small and despite the observed statistically significant results, it is possible that our findings are due to chance alone. Other factors affecting polyamine metabolism which were not accounted for in the present study may explain our observations. For example, aspirin activates polyamine acetylation and export and in association with the Odc1 A-allele to reduce cell and tissue polyamine contents.[12],[23],[31] NSAIDs have been shown associated with prolonged survival outcomes in nonmetastatic CRC cases,[15],[32] particularly among those consuming low levels of meat consumption.[16] Thus, aspirin, NSAIDs, or other concomitant medications or exposures (which are not available in the present study) may contribute to the observed risk estimates. Another limitation is that meat consumption was used as a surrogate for dietary polyamines, as no polyamine dietary database exists for this study. Also, information on cooking methods was unavailable, which may have relevance to the study outcomes. Given these important limitations, we consider the results presented here to be hypothesis-generating.
Conclusions
In summary, we have observed a statistically significant interaction between meat intake and Odc1 + 316 SNP with regard to CRC-specific mortality among CRC cases. Our findings suggest roles for meat consumption and genetic polymorphism at Odc1 on CRC progression. Furthermore, they may contribute to our ability to assess risk of CRC progression, with the ultimate goal of directing patient-specific pharmacogenetic risk-stratification, surveillance monitoring, and informing novel-targeted approaches to secondary and tertiary CRC prevention.
References
| 1. | Thomas T, Thomas TJ. Polyamine metabolism and cancer. J Cell Mol Med 2003; 7 :113-26.  [PUBMED] |
| 2. | Giardiello FM, Hamilton SR, Hylind LM, Yang VW, Tamez P, Casero RA Jr. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res 1997;57:199-201.  [PUBMED] |
| 3. | Fultz KE, Gerner EW. APC-dependent regulation of ornithine decarboxylase in human colon tumor cells. Mol Carcinog 2002;34:10-8.  [PUBMED] |
| 4. | Erdman SH, Ignatenko NA, Powell MB, Blohm-Mangone KA, Holubec H, Guillén-Rodriguez JM, et al. APC-dependent changes in expression of genes influencing polyamine metabolism, and consequences for gastrointestinal carcinogenesis, in the Min mouse. Carcinogenesis 1999;20:1709-13.  |
| 5. | Babbar N, Ignatenko NA, Casero RA Jr, Gerner EW. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem 2003;278:47762-75.  [PUBMED] |
| 6. | Meyskens FL Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32-8.  [PUBMED] |
| 7. | Thompson PA, Wertheim BC, Zell JA, Chen WP, McLaren CE, LaFleur BJ, et al. Levels of rectal mucosal polyamines and prostaglandin E2 predict ability of DFMO and sulindac to prevent colorectal adenoma. Gastroenterology 2010;139:797-805, 805.e1.  |
| 8. | McLaren CE, Fujikawa-Brooks S, Chen WP, Gillen DL, Pelot D, Gerner EW, et al. Longitudinal assessment of air conduction audiograms in a phase III clinical trial of difluoromethylornithine and sulindac for prevention of sporadic colorectal adenomas. Cancer Prev Res (Phila) 2008;1:514-21.  [PUBMED] |
| 9. | Zell JA, McLaren CE, Chen WP, Thompson PA, Gerner EW, Meyskens FL. Ornithine decarboxylase-1 polymorphism, chemoprevention with eflornithine and sulindac, and outcomes among colorectal adenoma patients. J Natl Cancer Inst 2010;102:1513-6.  [PUBMED] |
| 10. | Zell JA, Pelot D, Chen WP, McLaren CE, Gerner EW, Meyskens FL. Risk of cardiovascular events in a randomized placebo-controlled, double-blind trial of difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas. Cancer Prev Res (Phila) 2009;2:209-12.  [PUBMED] |
| 11. | Zell JA, Ziogas A, Ignatenko N, Honda J, Qu N, Bobbs AS, et al. Associations of a polymorphism in the ornithine decarboxylase gene with colorectal cancer survival. Clin Cancer Res 2009;15:6208-16.  [PUBMED] |
| 12. | Gerner EW, Meyskens FL Jr. Polyamines and cancer: Old molecules, new understanding. Nat Rev Cancer 2004;4:781-92.  [PUBMED] |
| 13. | Zell JA, Ignatenko NA, Yerushalmi HF, Ziogas A, Besselsen DG, Gerner EW, et al. Risk and risk reduction involving arginine intake and meat consumption in colorectal tumorigenesis and survival. Int J Cancer 2007;120:459-68.  [PUBMED] |
| 14. | Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA 2007;298:754-64.  [PUBMED] |
| 15. | Zell JA, Ziogas A, Bernstein L, Clarke CA, Deapen D, Largent JA, et al. Nonsteroidal anti-inflammatory drugs: Effects on mortality after colorectal cancer diagnosis. Cancer 2009;115:5662-71.  [PUBMED] |
| 16. | Zell JA, Ziogas A, Bernstein L, Clarke CA, Deapen D, Largent JA, et al. Meat consumption, nonsteroidal anti-inflammatory drug use, and mortality among colorectal cancer patients in the California Teachers Study. Cancer Prev Res (Phila) 2010;3:865-75.  [PUBMED] |
| 17. | Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: Dose-response meta-analysis of epidemiological studies. Int J Cancer 2002;98:241-56.  [PUBMED] |
| 18. | Peel DJ, Ziogas A, Fox EA, Gildea M, Laham B, Clements E, et al. Characterization of hereditary nonpolyposis colorectal cancer families from a population-based series of cases. J Natl Cancer Inst 2000;92:1517- 22.  [PUBMED] |
| 19. | Zell JA, Honda J, Ziogas A, Anton-Culver H. Survival after colorectal cancer diagnosis is associated with colorectal cancer family history. Cancer Epidemiol Biomarkers Prev 2008;17:3134-40.  [PUBMED] |
| 20. | Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev 2008;17:1950-62.  [PUBMED] |
| 21. | Ziogas A, Anton-Culver H. Validation of family history data in cancer family registries. Am J Prev Med 2003;24:190-8.  [PUBMED] |
| 22. | Guo Y, Harris RB, Rosson D, Boorman D, O′Brien TG. Functional analysis of human ornithine decarboxylase alleles. Cancer Res 2000;60:6314-7.  [PUBMED] |
| 23. | Martinez ME, O′Brien TG, Fultz KE, Babbar N, Yerushalmi H, Qu N, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci U S A 2003;100:7859-64.  [PUBMED] |
| 24. | Barry EL, Baron JA, Bhat S, Grau MV, Burke CA, Sandler RS, et al. Ornithine decarboxylase polymorphism modification of response to aspirin treatment for colorectal adenoma prevention. J Natl Cancer Inst 2006;98:1494-500.  [PUBMED] |
| 25. | Hubner RA, Muir KR, Liu JF, Logan RF, Grainge MJ, Houlston RS, et al. Ornithine decarboxylase G316A genotype is prognostic for colorectal adenoma recurrence and predicts efficacy of aspirin chemoprevention. Clin Cancer Res 2008;14:2303-9.  |
| 26. | Zoumas-Morse C, Rock CL, Quintana EL, Neuhouser ML, Gerner EW, Meyskens FL Jr. Development of a polyamine database for assessing dietary intake. J Am Diet Assoc 2007;107:1024-7.  [PUBMED] |
| 27. | Raj KP, Zell JA, Rock CL, McLaren CE, Zoumas-Morse C, Gerner EW,et al. Role of dietary polyamines in a phase III clinical trial of DFMO and sulindac for prevention of metachronous colorectal adenomas: A potential target for colon cancer chemoprevention. J Clin Oncol 2010;28: Abstract Number 1523.  |
| 28. | S0820, A double-blind placebo-controlled trial of eflornithine and sulindac to prevent recurrence of high risk adenomas and second primary colorectal cancers in patients with stage 0-III colon cancer. http://www.clinicaltrials.gov/ct2/show/NCT01349881. [Last accessed 2012 Sept 27].  |
| 29. | Barry EL, Mott LA, Sandler RS, Ahnen DJ, Baron JA. Variants downstream of the ornithine decarboxylase gene influence risk of colorectal adenoma and aspirin chemoprevention. Cancer Prev Res (Phila) 2011;4:2072-82.  [PUBMED] |
| 30. | Hughes DJ, Hlavatá I, Soucek P, Pardini B, Naccarati A, Vodickova L, et al. Ornithine decarboxylase G316A genotype and colorectal cancer risk. Colorectal Dis 2011;13:860-4.  |
| 31. | Babbar N, Gerner EW, Casero RA Jr. Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem J 2006;394:317-24.  [PUBMED] |
| 32. | Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA 2009;302:649-58.  [PUBMED] |
Authors
Dr. Jason Zell, Department of Epidemiology, 224 Irvine Hall, University of California, Irvine, CA, 92697, USA.
Dr. Bruce S. Lin, Division of Hematology/Oncology, Department of Medicine, UC Irvine Medical Center, Orange, CA 92868, USA.
Dr. Argyrios Ziogas, Department of Epidemiology, School of Medicine, UC Irvine, Irvine, CA 92697, USA.
Dr. Hoda Anton-Culver, Department of Epidemiology, School of Medicine, UC Irvine, Irvine, CA 92697, USA.
Figures
Tables
