F Lalfamkima1, GL Georgeno2, N Koteswara Rao3, Rajkumar Selvakumar4, Vimal Joseph Devadoss2, Niroshini Rajaram5, Shomaila Farid6, T Lalchhuanawma1, Abhishek Singh Nayyar7
1 Consultant Dental Surgeon, Selection Grade, Dental Department, Civil Hospital, Aizawl, Mizoram, India
2 Department of Oral and Maxillofacial Surgery, Rajas Dental College and Hospital, Kavalkinaru, Tirunelveli, Tamil Nadu, India
3 Department of Oral and Maxillofacial Surgery, Drs Sudha and Nageswara Rao Siddhartha Institute of Dental Sciences, Chinoutpalli, Gannavaram Mandal, Krishna District, Andhra Pradesh, India
4 Department of Oral and Maxillofacial Surgery, Rajah Muthiah Dental College and Hospital, Annamalai University, Annamalai Nagar, Cuddalore, Tamil Nadu, India
5 Department of Oral Pathology and Microbiology, RVS Dental College and Hospital, Sulur, Coimbatore, Tamil Nadu, India
6 Consultant, Division of Infection Prevention and Control, Infection Control Department, Security Forces Specialized Health Center, Taif, Kingdom of Saudi Arabia
7 Department of Oral Medicine and Radiology, Saraswati Dhanwantari Dental College and Hospital and Post-graduate Research Institute, Parbhani, Maharashtra, India
| Date of Submission | 04-Dec-2020 |
| Date of Decision | 02-Feb-2021 |
| Date of Acceptance | 19-Feb-2021 |
| Date of Web Publication | 13-Apr-2021 |
Correspondence Address:
T Lalchhuanawma
Consultant Dental Surgeon, Selection Grade, Dental Department, Civil Hospital, Aizawl, Mizoram
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_27_20
Abstract
CONTEXT AND AIM: The inaccuracies in clinical examination have been well documented, while advanced imaging modalities, including computed tomography and magnetic resonance imaging (MRI), have been shown to have superior diagnostic accuracy in detecting occult and nodal metastasis. The aim of the present study was to identify as well as evaluate the inaccuracies in clinical examination and of clinical diagnostic criteria in known cases of oral squamous cell carcinomas (OSCCs) with the help of MRI.
MATERIALS AND METHODS: A total of 24 patients attending as outpatients were included in the study, while clinically diagnosed and histopathologically proven cases of OSCC were examined clinically and then subjected to advanced imaging with the help of MRI.
STATISTICAL ANALYSIS USED: Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL, USA), while paired t-test was performed for evaluating the size of tumor and lymph node recorded on clinical and imaging findings. A P < 0.05 was considered statistically significant.
RESULTS: Detection of tumor size and lymph node metastasis was found to be higher in case of MRI than when accomplished by clinical staging alone, while paired t-test values for difference in results were found to be statistically significant (P < 0.05).
CONCLUSIONS: The present study showed that clinical diagnostic criteria alone were not sufficient and reliable for detecting metastatic lymphadenopathy, highlighting the significance of advanced imaging modalities such as MRI for an efficient preoperative diagnostic workup, as well a tool for planning treatment in patients with OSCCs.
Keywords: Magnetic resonance imaging, metastasis, multimodal imaging, oral squamous cell carcinoma.
| How to cite this article: Lalfamkima F, Georgeno G L, Rao N K, Selvakumar R, Devadoss VJ, Rajaram N, Farid S, Lalchhuanawma T, Nayyar AS. Clinical diagnostic criteria versus advanced imaging in prediction of cervical lymph node metastasis in oral squamous cell carcinomas: A magnetic resonance imaging based study. J Carcinog 2021;20:3 |
| How to cite this URL: Lalfamkima F, Georgeno G L, Rao N K, Selvakumar R, Devadoss VJ, Rajaram N, Farid S, Lalchhuanawma T, Nayyar AS. Clinical diagnostic criteria versus advanced imaging in prediction of cervical lymph node metastasis in oral squamous cell carcinomas: A magnetic resonance imaging based study. J Carcinog [serial online] 2021 [cited 2021 Oct 13];20:3. Available from: https://carcinogenesis.com/text.asp?2021/20/1/3/313654 |
Introduction
Oral squamous cell carcinoma (OSCC) is the sixth most common cause of cancer-related deaths worldwide.[1] In the Indian scenario, oral cancer is the second most common cancer related to deaths.[2] The presence of metastatic cervical lymphadenopathy is of particular importance, as with every single nodal metastasis, survival of the patient is reduced by half.[3] Thus, regional metastasis is one of the most important factors in deciding for prognosis and planning treatment in patients with OSCCs.[4],[5],[6] The inaccuracies in clinical examination have, also, been well documented, while advanced imaging modalities including computed tomography (CT) and magnetic resonance imaging (MRI) have been shown to have superior diagnostic accuracy in detecting occult and nodal metastasis.[7],[8],[9],[10],[11] Most commonly used tumor (T), node (N), and metastasis (M) classification (TNM classification) system fails to define the exact size and measurements of tumor including diameter, length, width, area, volume, and tumor thickness as well as clinical issues related to it.[12],[13] An appropriate management of OSCC cases requires a thorough understanding of factors affecting incidence, patterns, and prognostic implications of nodal metastasis.[6] Identification of such prognostic factors could constitute one of the important keys to reduce mortality, morbidity, and most importantly, the recurrences in treated patients. The prognosis of patients with OSCCs that are treated early, in initial phases of malignancy, is found to be better because cure is achieved with less complex and lesser aggressive measures than what is obtainable in case of lesions that have reached advanced stages or metastases. Despite higher incidence and also higher mortality rates reported from India, no study describing diagnostic accuracy of important prognostic factors such as tumor thickness related to lymph nodal metastasis using modern imaging techniques has been documented.[14] Furthermore, a number of studies have identified that among all aspects of tumor size studied, tumor thickness can be an important indicator in prediction of nodal metastasis as well as local recurrences and survival in patients with OSCC.[15],[16],[17],[18],[19] Several such studies have been conducted on postoperative, resected specimens, while there are very few studies reported which were conducted preoperatively. Further complicating this situation is the observation that tumor size involving different areas has been found to affect different levels of lymph nodes and thereby controls survival in a varied manner.[4],[5] Hence, an accurate preoperative assessment of tumor size, in its all possible dimensions, becomes an essential component in optimizing treatment algorithm.[20] MRI is a powerful imaging tool for cross-sectional analysis of head and neck. This is, especially, true with regard to oropharyngeal neoplasms wherein soft tissue spread, nodal disease, peri-neural invasions, and osseous involvement significantly alter treatment plan and prognosis.[20],[21] The aim of the present study was to identify as well as evaluate the inaccuracies in clinical examination and of clinical diagnostic criteria in known cases of OSCCs with the help of MRI.
Materials and Methods
A total of 24 patients including 11 patients with OSCC involving alveolus, six patients with OSCC involving tongue, and seven patients with OSCC involving buccal mucosa attending as outpatients were included in the study, while clinically diagnosed and histopathologically proven cases of OSCC were examined clinically and then subjected to advanced imaging with the help of MRI [Figure 1]. Patients with known systemic diseases, those who had received any previous treatment in the form of radiotherapy, surgery, or chemotherapy, those with known contraindications to MRI including patients with cardiac pacemaker, cochlear implants, and ocular and dental implants, and patients in whom gadolinium-based contrast agents were contraindicated were excluded. The study protocol was approved by the Institutional Ethics Committee via letter approval no. SDDC/IEC/07-39-2018, while written, informed consent was obtained from all patients who participated in study. All patients were, then, subjected to routine blood investigations, fine-needle aspiration cytology (FNAC) of the ipsilateral submandibular lymph node (Level I), and incisional biopsy of oral lesion to confirm the diagnosis. As part of prediagnostic workup, a thorough clinical examination was performed, while details were recorded in a specially devised pro forma. Complete lymph nodal examination was carried out. The armamentarium used for MRI examination included 1.5 Tesla Magnetom Avanto Systems (Siemens, Germany). All patients were asked to fast for 6 h before MRI examination, while specific sequences were obtained including axial T1-weighted, spin-echo images from face and neck region including maxilla and mandible wherein localizer was widened in cases of larger fields (TR/TE: 400–640 ms/10–14 ms; slice thickness: 4–7 mm; gap: 1–2 mm; field of view: 24–38 cm; NEX: 1–2; matrix: 256 × 192–256). Followed by this, axial T2-weighted, fast spin-echo images of the face and neck (TR/TE: 4000–6000 ms/90–110 ms; echo-train length: 8; slice thickness: 4–7 mm; gap: 1–2 mm; field of view: 24–38 cm; NEX: 2; matrix: 512 × 256), sagittal T2-weighted, fast spin-echo images from canthomeatal line to supraclavicular level (TR/TE: 4000–6000 ms/90–110 ms; echo-train length: 8; slice thickness: 4–7 mm; gap: 1–2 mm; field of view: 24–32 cm, NEX: 2; matrix: 512 × 256), and unenhanced and enhanced fat-suppressed, coronal T1-weighted images (TR/TE: 126 ms/2.47 ms; flip angle: 70°; slice thickness: 4–7 mm; gap: 1–2 mm; field of view 30–36 cm; NEX: 2–4; matrix: 256 × 192–256; spectral fat suppression) were obtained. Contrast-enhanced images were obtained after intravenous injection of 0.1 mmol/kg body weight of gadobenate dimeglumine (Multihance; Bracco, Milan, Italy). Postcontrast images were obtained in two planes. Additional sequences were, also, taken in few patients as per the requirement while doing procedure. With the help of these sequences, tumor size was assessed in terms of diameter, length, width, area, volume, and tumor thickness, while cervical lymph nodes were assessed in terms of their size, number, and grouping or confluence and associated changes, if any. MRI parameters were analyzed by three expert radiologists who were blinded to clinical findings. Tumor size in all three dimensions [Figure 2] was assessed for its relation with lymph node metastasis. For measuring tumor thickness, a horizontal line joining two tumor–mucosa junctions was drawn as the reference line and tumor thickness was measured by drawing perpendicular lines from the said reference line to the point of maximal tumor projection and invasion as seen on MRI images and calculated as the greatest determined tumor thickness by adding these two parameters [Figure 3]. The diagnosis of lymph node metastasis was made based on specific imaging criteria on MRI including a size >8 mm and round shape. Size criterion was taken as standard and diagnostic reliability of all other variables was calculated in relation to it in prediction of cervical lymph node metastasis [Figure 4]. All patients were assessed for tumor size, lymph node metastasis, lymph nodal grouping [Figure 5], and peri-neural spread of tumor [Figure 6] and [Figure 7] using MRI, while diagnostic reliability for lymph node metastasis in relation to tumor thickness was calculated. The results obtained were, then, subjected to statistical analysis, while sensitivity, specificity, positive and negative predictive values, and accuracy were also calculated.
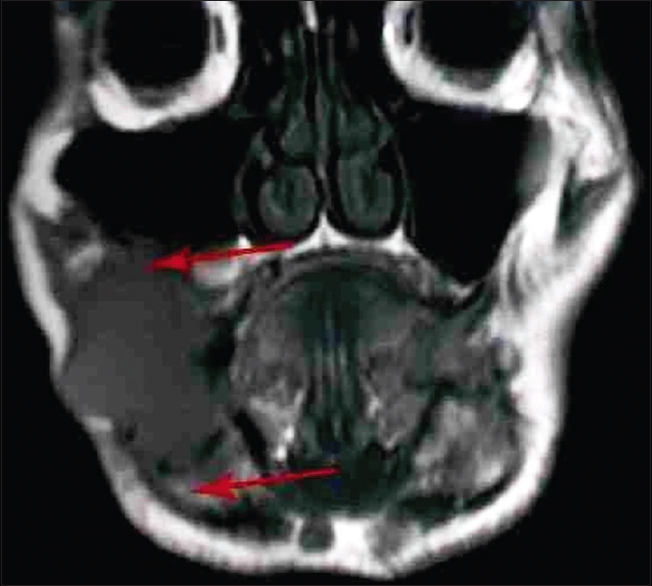 |
Figure 1: Magnetic resonance imaging image of a patient (coronal view) Click here to view |
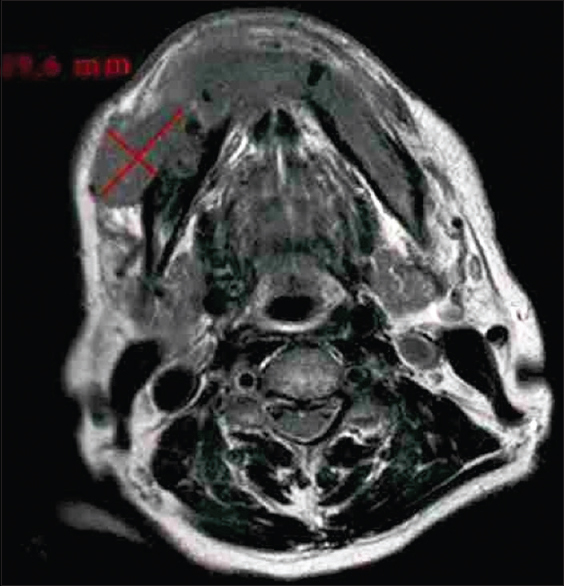 |
Figure 2: Tumor size on magnetic resonance imaging image (axial view) Click here to view |
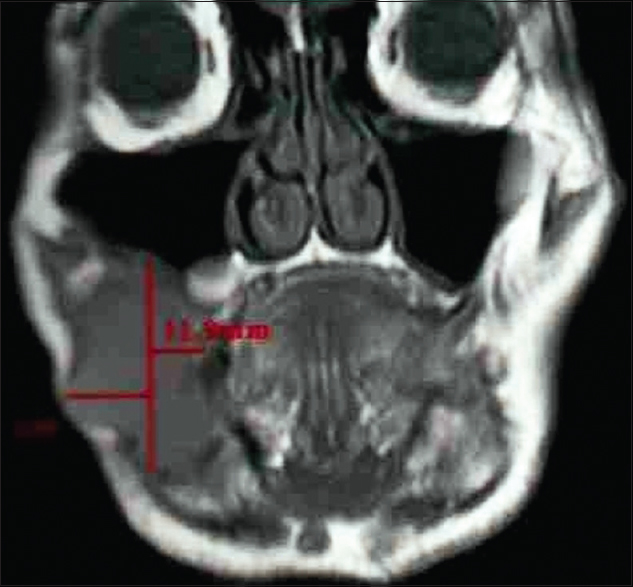 |
Figure 3: Tumor thickness on magnetic resonance imaging image (coronal view) Click here to view |
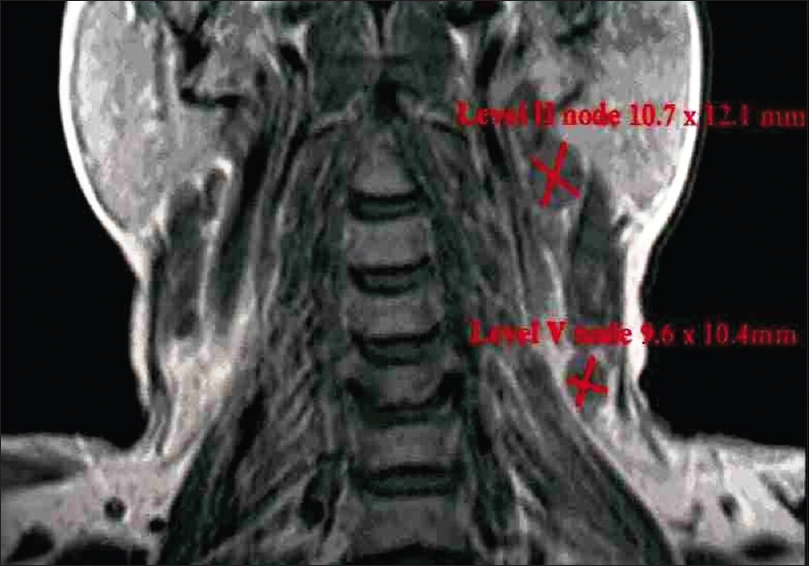 |
Figure 4: Lymph node size on magnetic resonance imaging image (coronal view) Click here to view |
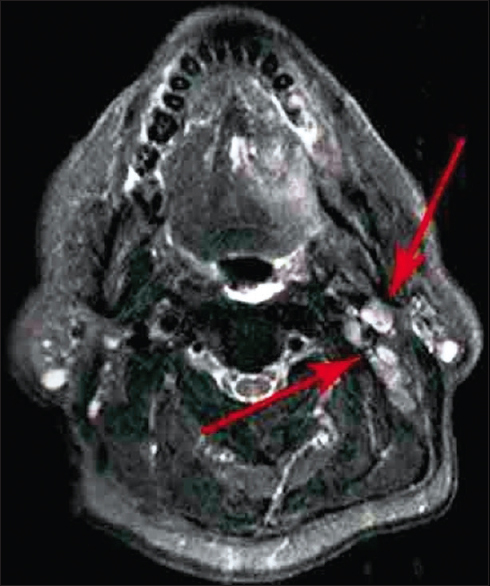 |
Figure 5: Lymph node grouping on magnetic resonance imaging image (axial view) Click here to view |
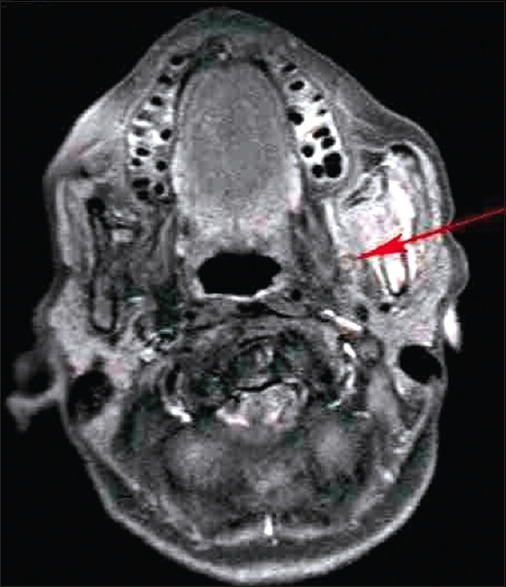 |
Figure 6: Peri-neural spread on magnetic resonance imaging image (axial view) Click here to view |
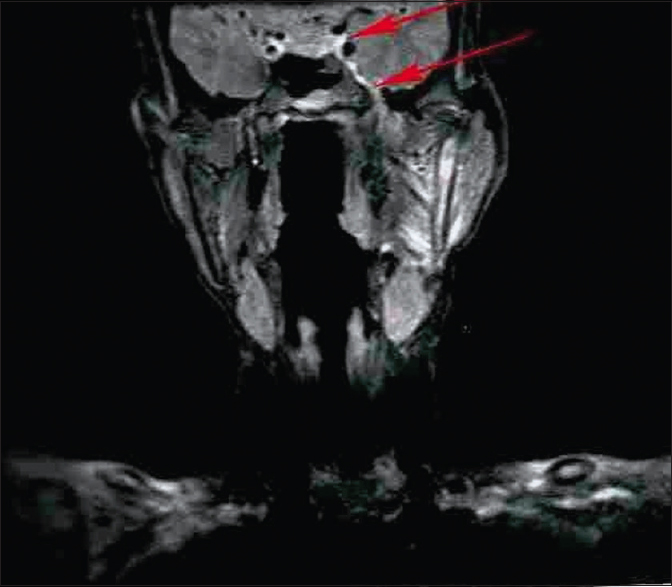 |
Figure 7: Peri-neural spread on magnetic resonance imaging image (coronal view) Click here to view |
Statistical analysis used
Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL, USA), while paired t-test was performed for evaluating size of tumor and lymph node recorded on clinical and imaging findings. A P < 0.05 was considered statistically significant.
Results
The age of the patients in the study ranged from 37 to 84 years, with a mean age of 60 years. The distribution of cases according to age and gender is shown in [Table 1] while by site, tumor differentiation, and FNAC-positive lymph nodes in [Table 2]. Out of the 24 patients included in study with palpable lymph nodes, 20 lymph nodes were found positive for FNAC confirming metastatic spread [Table 2]. [Table 3] reveals the distribution of cases by clinical and imaging staging, wherein it was observed that five cases were upgraded from T2 to T3, two cases from T3 to T4, while only one patient was downgraded from T2 to T1 on MRI examination. On evaluating lymph nodes, five cases (20.83%) were upgraded from N0 to N1 while one case (4.17%) was upgraded from N2a to N2b as observed on MRI. [Table 4] reveals the relationship between tumor thickness and lymph node metastasis for all levels of lymph nodes wherein tumor thickness observed in the present study varied from 1.4 to 5.6 cm, while positive cases for lymph node metastasis were found to be more when tumor thickness was >3 cm in case of both Level I (88.89%) and all levels (81.25%) of lymph nodes, with the corresponding values being statistically significant (P < 0.05). [Table 5] shows the diagnostic reliability of different cutoff thickness in relation to lymph node metastasis for all levels of lymph nodes, wherein the diagnostic accuracy for the detection of lymph nodal metastasis in relation to tumor thickness of 2 cm, 3 cm, and 4 cm was reported to be 40.0%, 32.0%, and 76.0%, respectively, for Level I and 36.96%, 41.30%, and 69.57% for all levels of lymph nodes. [Table 6] shows the relationship between lymph node number and metastasis, while [Table 7] shows the diagnostic reliability for lymph node metastasis in relation to lymph node number, grouping, and associated changes for all levels of lymph nodes. The diagnostic accuracy for lymph node metastasis in relation to lymph node number, grouping, and associated changes was found to be 56.0%, 56.0%, and 52.0%, respectively, for Level I while 60.87%, 58.7%, and 50.0% for all levels of lymph nodes. [Table 8] shows comparison of clinical and imaging staging with varied tumor-related parameters and lymph node size (in mm), wherein on analysis, it was found that tumor sizes identified on imaging with a mean of 4.04 mm for length and 3.08 mm for width, respectively, were significantly higher than as assessed by clinical staging with a mean of 2.94 mm and 2.47 mm for length and width, respectively. Similarly, the size of lymph nodes identified on imaging with a mean of 11.45 mm was found to be higher than as assessed by clinical staging with a mean of 8.00 mm, with the corresponding values being statistically significant (P < 0.05). [Table 9] reveals overall diagnostic reliability of MRI staging for lymph node metastasis, wherein it could be observed that the overall specificity, sensitivity, and positive and negative predictive values as observed on imaging were found to be 100.0%, 75.0%, 72.73%, and 100.0%, respectively, while the overall accuracy achieved was 85.0%.
 |
Table 1: Distribution of cases by age and gender Click here to view |
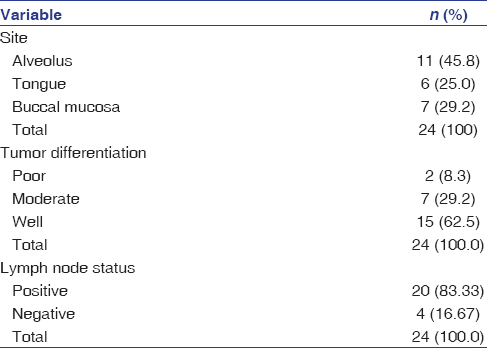 |
Table 2: Distribution of cases by site, tumor differentiation and fine-needle aspiration cytology-positive lymph nodes Click here to view |
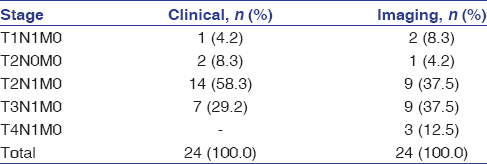 |
Table 3: Distribution of cases by clinical and imaging staging Click here to view |
| Table 4: Relationship between tumor thickness and lymph node metastasis for all levels of lymph nodes Click here to view |
| Table 5: Diagnostic reliability of different cut-off thickness in relation to lymph node metastasis for all levels of lymph nodes Click here to view |
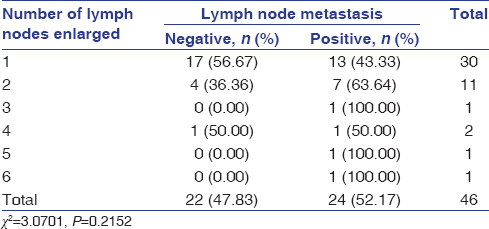 |
Table 6: Relationship between lymph node number and metastasis Click here to view |
| Table 7: Diagnostic reliability for lymph node metastasis in relation to lymph node number, grouping and associated changes for all levels of lymph nodes Click here to view |
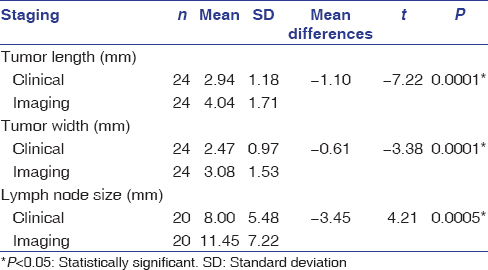 |
Table 8: Comparison of clinical and imaging staging with varied tumor-related parameters and lymph node size (mm) Click here to view |
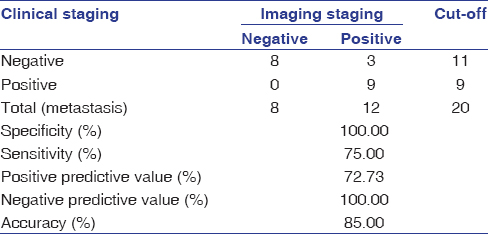 |
Table 9: Overall diagnostic reliability of magnetic resonance imaging staging for lymph node metastasis Click here to view |
Discussion
Although CT remains the most widely used imaging modality, it delivers a very high dose of radiation to patients, while on the contrary, MRI is a safer and new procedure for the evaluation of OSCCs and associated lymphadenopathy which is preferred for its excellent soft tissue resolution, especially in three-dimensional visualizations.[21],[22] Ultrasonography, though used for soft tissue imaging, is a highly operator-dependent imaging modality.[16] Although MRI is considered to be a costly imaging modality till date, its use for accurate preoperative evaluation of tumor size and metastatic lymphadenopathy can significantly decrease morbidity in OSCC patients.[4] The use of MRI, in the head and neck region, is, also, supported by the facts that orofacial tissues have variable amount of fat in different regions and tumor extent can be clearly seen on MRI images by fat-suppression techniques. Moreover, MRI images are free from metal streak artifacts seen on CT images.[22] The major limitations of MRI, however, include its restricted availability and difficulty in performing MRI scan in claustrophobic, old, and uncooperative patients. MRI is, also, contraindicated in patients with ferromagnetic-based implants, including cardiac pacemakers and aneurysmal bone and vascular clips.[23],[24],[25]
Inaccuracies in clinical examination have been well documented. In the present study, tumor size as evaluated by TNM staging based on MRI was found to be higher for most of the cases as compared to that documented on clinical examination. Only one case was downgraded from T2 to T1 on MRI examination, the probable reason for which could be that the largest tumor dimension got obscured within 4–7 mm slice thickness used for acquisition of MRI images. Furthermore, nodal involvement was found to be more when evaluated with MRI as compared to clinical staging, wherein 5 cases (20.83%) were upgraded from N0 to N1 while one case (4.17%) was upgraded from N2a to N2b as observed on MRI. It was, also, evident from findings of the present study that whenever tumor sizes were larger, widespread nodal metastases were noticed while a significant difference was noted in tumor sizes as identified on imaging than as assessed by clinical staging. Similarly, sizes of lymph nodes identified on imaging were found to be higher than as assessed by clinical staging, with the corresponding values being statistically significant (P < 0.05). The results of the present study revealed the overall specificity, sensitivity, and positive and negative predictive values as observed on imaging to be 100.0%, 75.0%, 72.73%, and 100.0%, respectively, while the overall accuracy achieved was 85.0%.
Bipat et al.,[26] after reviewing 57 high-quality studies, concluded that sensitivity of MRI was higher (60%) as compared to CT (43%) for detecting metastatic lymph nodal involvements. Hayashi et al.,[27] however, reported an accuracy of 75% with a cutoff value of 5 mm tumor thickness for subsequent lymph node metastasis when using CT. In the present study, a diagnostic accuracy of 76.0% was achieved with a cutoff value of 4 cm tumor thickness for Level I while 69.57% for all levels of lymph nodes when using MRI. Diagnostic accuracy reported by Hayashi et al.[27] using CT and the present study using MRI is comparable; however, disadvantages of CT have to be noted. Significantly increased risk for cervical lymph nodal metastasis was, also, noticed by Fukano et al.[15] and O-Charoenrat et al.[16] with tumor thickness of >5 mm for oral and oropharyngeal carcinomas. Furthermore, it is true that tumor deposits within a node are only microscopic and detection of metastasis is usually difficult at microscopic level; however, when metastases are large enough to be detected by CT or MRI, criteria are necessary to stage the disease.[25]
There are no clear recommendations regarding size criterion and different values have also been studied.[28] The present study used a size criterion of 8 mm as standard for predicting lymph node metastasis in analyzing accuracy of other imaging criteria. There are very few studies that have considered number of lymph nodes involved as an imaging criterion to predict prognosis in OSCCs, though literature review reveals worse prognosis whenever more number of lymph nodes were involved in given carcinoma.[29] In the present study, diagnostic accuracy for lymph node metastasis in relation to lymph node number, grouping, and associated changes was found to be 56.0%, 56.0%, and 52.0%, respectively, for Level I while 60.87%, 58.7%, and 50.0% for all levels of lymph nodes. Furthermore, specificity values of 83.33% and 81.82% were observed in the present study in relation to grouping of lymph nodes as a diagnostic criterion for predicting lymph nodal metastasis; the finding, however, was contradictory to the finding of the study conducted by van den Brekel et al.,[28] who stated that grouping criterion did not increase specificity for the detection of lymph node metastasis. Furthermore, in the present study, a sensitivity of 4.17%–7.69% while a specificity of 100.0% for peri-neural spread as imaging criterion were reported on MRI, though relatively higher values of sensitivity and specificity were observed in the study conducted by Hanna et al.,[30] with a reported sensitivity of 100% and specificity of 85% using MRI and 88% and 89%, respectively, using CT. The low value of sensitivity observed in the present study, however, can be attributed to the fact that only one case in the present study was found to have associated changes with lymph node metastasis in the form of peri-neural spread on MRI.
Clinical examination remains the worldwide standard and the very first step for initial screening and evaluation of OSCCs; however, it fails to define exact tumor size and extent and associated lymph nodal metastasis. Furthermore, surface size is the main verifiable clinical parameter available before treatment because nodal spread estimates are often erroneous while biopsies, also, often do not reflect the whole tumor histology.[31] Furthermore, thickness of tumor, or its volume, even if, assessed before treatment, can only be a rough estimate which are not mentioned in the official TNM manual.[32],[33] In patients with head and neck carcinomas, prognosis is usually obtained based on TNM classification which is based on the clinical criteria and is highly useful, especially to assess the essential features such as local extension, regional dissemination, and distant metastasis.[34],[35] However, this classification lacks a clear idea about aggressiveness of tumor and inflammatory versus metastatic lymph nodal enlargements. Clinical impression of nodal metastasis on the first examination is often overestimated, and this might be because of the regional inflammatory reaction to a metastatic node. In addition, histopathological examination, which is the gold standard, is currently being done postoperatively only. In such circumstances, a study correlating MRI findings and histologic tumor thickness and associated lymph nodal metastasis in the assessment of OSCCs gives a clear view about this aspect. One such study done by Lam et al.[17] in oral tongue carcinomas stated that radiologic tumor thickness as measured on contrast-enhanced MRI images had a significant correlation with histologic tumor thickness.
OSCCs may have different behaviors in different areas of the oral mucosa. Thus, traditional TNM clinical staging fails to predict exact prognosis. It has, also, been reported previously that when dealing with primary tumors, advanced cases with infiltration of adjacent structures were hardly assessed on clinical examination with the percentage of accuracy found to be higher when CT and MRI were used for classification and staging of tumor as compared to TNM clinical staging alone.[36] In the present study, only 40% of cases were found to be true positive for detecting lymph nodal metastasis using clinical diagnostic criteria, while the numbers were 55% using imaging criteria. Thus, imaging criteria were found to be more accurate than clinical diagnostic criteria. This might, also, be because none of the available imaging criteria for lymph nodal metastasis are standardized till date.
It is of further interest to note that few cases in the present study, particularly tongue and buccal mucosa, showed involvement of Level III and IV lymph nodes on MRI but were clinically occult (N0), thereby emphasizing the significance of MRI in providing useful information regarding treatment planning in such cases. By detecting some otherwise clinically occult lymphadenopathy, MRI may have increased sensitivity for detecting positive nodes and consequently may decrease risk of occult metastasis which might be of extreme significance in decision-making as treatment strategies for N0 and N1 or N2 necks differ distinctly.[37] The comparatively lower sensitivity and specificity reported in the present study might be attributed to the reason that cases involving three different regions of the oral cavity were included which might have different patterns of nodal metastasis. Furthermore, OSCCs involving alveolus do not spread to lymph nodes as rapidly as the ones involving tongue and buccal mucosa.[4],[5],[16],[27]
Conclusions
The present study revealed that clinical diagnostic criteria alone were not sufficient and reliable for detecting metastatic lymphadenopathy, highlighting the significance of advanced imaging modalities such as MRI for an efficient preoperative diagnostic workup as well as a tool for planning treatment in patients with practically incurable OSCCs. Based on the findings of present study, it was, also, observed that whenever tumor size, particularly, tumor thickness of >4 cm, was observed, cervical lymph nodal metastasis could be predicted accurately which, in turn, had a significant impact on prognosis. The study did encounter a limitation in the form of enhancement of inflamed and edematous soft tissues on MRI, especially on T2-weighted, spin-echo images, but this was seen as a common drawback in previous studies as well, related to MRI.
Future research directions
Although MRI is an extremely useful advanced imaging modality for an efficient preoperative diagnostic workup and as a tool for planning treatment, it might not provide equally useful information postsurgery and/or radiotherapy when anatomical planes are distorted. In such cases, fluorodeoxyglucose-based positron emission tomography may be used to get necessary information regarding residual/recurrent malignant and metastatic foci and associated lymphadenopathy.[20] Furthermore, in the future, newer technologies applied to MRI including MRI microimaging and functional MRI might give more advanced outcomes with higher diagnostic and predictive accuracies and help in improving survival of affected patients.[38]
Acknowledgment
We would acknowledge all the patients who contributed in the study, without whom, this study would not have been feasible.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. |
Po Wing Yuen A, Lam KY, Lam LK, Ho CM, Wong A, Chow TL, et al. Prognostic factors of clinically Stage I and II oral tongue carcinoma – A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck 2002;24:513-20.
 |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. | |
| 25. | |
| 26. | |
| 27. | |
| 28. | |
| 29. | |
| 30. | |
| 31. | |
| 32. | |
| 33. | |
| 34. | |
| 35. | |
| 36. | |
| 37. | |
| 38. |
Figures
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6], [Figure 7]
Tables
[Table 1], [Table 2], [Table 3], [Table 4], [Table 5], [Table 6], [Table 7], [Table 8], [Table 9]
