Preeti Singh1, Dominic Augustine1, Roopa S Rao1, Shankargouda Patil2, Kamran Habib Awan3, Samudrala Venkatesiah Sowmya1, Vanishri C Haragannavar1, Kavitha Prasad4
1Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences, Bengaluru, Karnataka, India
2Department of Maxillofacial Surgery and Diagnostic Sciences, College of Dentistry, Jazan University, Jazan, Saudi Arabia
3College of Dental Medicine, Roseman University of Health Sciences, South Jordan, Utah, USA
4Department of Oral and Maxillofacial Surgery, Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences, Bengaluru, Karnataka, India
| Date of Submission | 16-May-2020 |
| Date of Decision | 30-Aug-2020 |
| Date of Acceptance | 05-Dec-2020 |
| Date of Web Publication | 23-Sep-2021 |
Correspondence Address:
Dominic Augustine
Department of Oral Pathology and Microbiology, Faculty of Dental Sciences, MS Ramaiah University of Applied Sciences, MSR Nagar, Bengaluru – 560 054, Karnataka
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_14_20
Abstract
Targeting cancer stem cell (CSC) subpopulation within the tumor remains an obstacle for specific therapy in head-and-neck squamous cell carcinoma (HNSCC). Few studies in the literature describe a panel of stem cell makers, however a distinct panel has not been put forth. This systematic review aims to enhance the knowledge of additional markers to accurately relate their expression to tumorigenesis, metastasis, and therapy resistance. Databases, including PubMed, Google Scholar, Ebsco, and Science Direct, were searched from 2010 to 2017 using various combinations of the following keywords: “Stem cell markers in HNSCC” and “chemoresistance and radioresistence in HNSCC.” Original experimental studies (both in vitro and in vivo) published in English considering stem cell markers in HNSCC, were considered and included. We excluded articles on tumors other than HNSCC, reviews, editorial letters, book chapters, opinions, and abstracts from the analyses. Forty-two articles were included, in which 13 types of stem cell markers were identified. The most commonly expressed CSC markers were CD44, aldehyde dehydrogenase, and CD133, which were responsible for tumorigenesis, self-renewal, and therapy resistance, whereas NANOG, SOX-2, and OCT-4 were involved in metastasis and invasion.Identification of an accurate panel of CSC markers is the need of the hour as nonspecificity of the current markers poses a problem. Further studies with a large sample size would help validate the role of these CSC markers in HNSCC. These CSC proteins can be developed as therapeutic targets for HNSCC therapy, making future treatment modality more specific and effective.
Keywords: Aldehyde dehydrogenase, cancer stem cells, CD133, CD44, NANOG, OCT-4, SOX-2, targeted therapy.
| How to cite this article: Singh P, Augustine D, Rao RS, Patil S, Awan KH, Sowmya SV, Haragannavar VC, Prasad K. Role of cancer stem cells in head-and-neck squamous cell carcinoma – A systematic review. J Carcinog 2021;20:12 |
| How to cite this URL: Singh P, Augustine D, Rao RS, Patil S, Awan KH, Sowmya SV, Haragannavar VC, Prasad K. Role of cancer stem cells in head-and-neck squamous cell carcinoma – A systematic review. J Carcinog [serial online] 2021 [cited 2021 Oct 13];20:12. Available from: https://carcinogenesis.com/text.asp?2021/20/1/12/326586 |
Introduction
Although CSCs form a very small proportion of the tumor cell population, they play a significant role in determining outcomes. Generally, CSCs refer to the cancer cells capable of self-renewal and differentiation, which makes them resistant to radiotherapy and chemotherapy.[1] CSCs have stem features like that of normal cells (NSCs) such as self-renewal, high proliferation abilities, high migration capacity, and drug resistance.[2] In disease progression, tumor initiation and metastasis, and treatment resistance in head-and-neck squamous cell carcinoma (HNSCC), CSCs play a vital role.[3] Even in a small spectrum of cells, existing in a tissue, CSCs can be clearly separated from other cells.[4]
At present, development of new therapeutic planning is hindered because of lack of suitable and reliable markers for identification of CSCs. Although the role of CSCs in the renewal and initiation of tumors has been discovered,[5] the association between CSCs and metastasis is yet to be rooted out. In stem cells and CSCs, similar features are found, such as activation of DNA-repair machinery and expression of drug transporter ABC. Understanding the biology of cancer stem cells (CSCs) with difference in biologic behaviors from NSCs will help in the identification of molecular targets for targeted therapy.[1],[2],[3],[4],[5] Furthermore, it has been reported that patients with high expression of CD44, aldehyde dehydrogenase (ALDH) 1, and SOX2 had worse prognosis.[6],[7],[8],[9],[10] The prognostic value of CSCs in HNSCC remains controversial. CSC therapy-resistance is because of adaption, quiescence, survival, damaged DNA repairing, and detoxification/multidrug resistance. Because these traits can be shared by CSCs from different malignancies, it is essential to know the nature of the underlying biology of these driving mechanisms.[8],[11],[12],[13],[14] A larger area of cells that proliferate symmetrically, with both daughter cells showing the stem cell phenotype, and CSCs vividly differ from adult cells. These cells have increased replicative potential resulting from mutations in stem/progenitor. CSCs and NSCs in adult somatic tissues show common features of slow cycling and self-renewal. It is still doubtful whether or not CSCs are fully dependent on the niche, like NSCs that vary in different types of tumors.[5],[10],[15]
Despite all studies and analyses done on CSCs, no single biomarker has been found to define the CSC population for HNSCC, exactly. A set of biomarkers are needed to accurately define this population for identification and targeted therapy. Targeting CSCs in HNSCC for chemoresistance, radioresistence, and immune evasion mechanism remains a cornerstone for novel adjunct therapies. The aim of the current chemotherapy and radiation treatment for HNSCC is to debulk the tumor, whereas the CSC hypothesis shows that the elimination of CSCs is the only way to treat cancer with high efficacy. This systematic review aims to identify a panel of existing HNSCC CSC markers that are involved in cancer progression, metastasis, treatment resistance, and prognosis. The review also explores the resistance mechanism of CSCs in HNSCC and outlines the differences between CSCs and NSCs.
Materials and Methods
Key question
A key question was constructed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). The question was Can a specific panel of CSC markers be identified that play a major role in tumorigenesis, metastasis, and therapy resistance. This systemic review was reported according to the PRISMA checklist (www.prisma.statement.com).[16] Patient, Intervention, Comparison, and Outcomes method as applicable in relation to the topic of the review is as follows:
- Patients: Individuals with HNSCC
- Intervention: Stem cell markers
- Comparison: Intercomparison between various stem cell makers for HNSCC
- Outcomes: Correlation of stem cell markers with tumorigenesis, metastasis, and therapy resistance.
Study design
A systematic review was done on studies which used the appropriate panel of surface antigens having stem cell-like property which play important role in tumorigenesis, metastasis, and resistance to therapy in HNSCC.
Inclusion criteria
- Full-length English articles were considered that focused on surface antigen having stem cell-like property with their role in the biological behavior of tumor
- Articles that emphasize the role of CSC in resistance to the therapy in HNSCC.
Exclusion criteria
- Tumors other than HNSCC
- Articles other than original research, such as reviews, letters, personal opinions, book chapters, and conference abstracts were excluded
- Insufficient information or results not individualized for HNSCC were excluded.
Data sources and strategy of search
PubMed, Google Scholar, Ebsco, Scopus, and ScienceDirect databases were used to search for appropriate articles using keywords (stem cell markers in HNSCC and chemoresistance and radioresistence in HNSCC) [Table 1]. The search included all articles published up to March 2017, across all databases, with no time restrictions. In addition, the reference lists of selected articles were checked for additional relevant study which could have been missed during electronic search.
| Table 1: Methodology employed for the review Click here to view |
Data collection process
The data collection was done in the following three steps:
- Evaluation of collected articles
- Shortlisting of articles which included CSC antigen
- Evaluation of methodology and assessment of results.
Included studies and information recorded were as follows:
- Author, year of publication, and countries
- Study characteristics (type of surface antigen of CSCs).
Assessing risk of bias
The Cochrane Collaboration tool (Higgins JPT, Green S et al., 2011) was applied to assess the risk of bias for randomized controlled trials. Bias was evaluated as a judgment (high, low, or unclear) for individual elements from seven domains. Risk of bias was assessed for each included study from six aspects namely (1) random sequence generation (selection bias), (2) allocation concealment (selection bias), (3) blinding of outcome assessment (detection bias), (4) incomplete outcome data (attrition bias), (5) selective reporting (reporting bias), and (6) other bias. Risk of bias was rated by two independent researchers (PS and DA). Disagreements were discussed and resolved by a third researcher (SVS).
Synthesis of results
The results of individual studies were summarized and the most appropriate surface antigen for CSCs was analyzed and grouped; pathways leading to therapy resistance were analyzed and summarized. Summarization of individual points of interests across the selected studies was carried out [Figure 1].
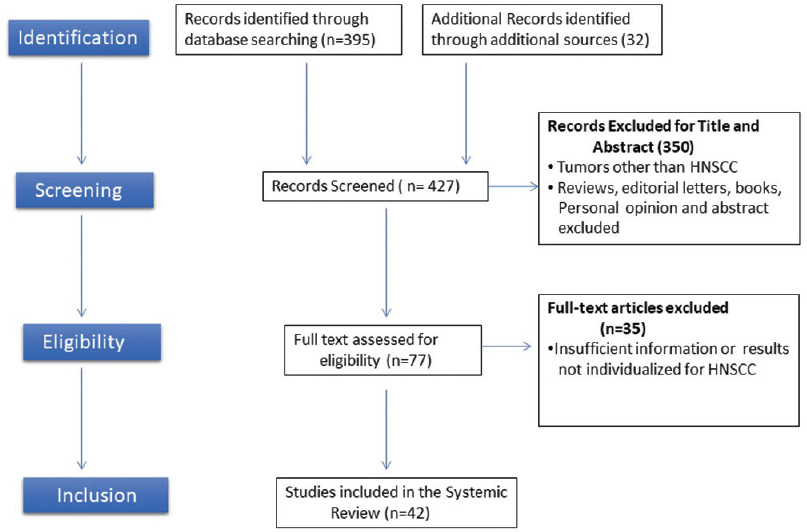 |
Figure 1: PRISMA flow diagram of studies selection Click here to view |
Results
Search results
On searching with the above-mentioned keywords, 427 search results were identified. However, these included review articles, short communications, and journal publications. Among those, 200 articles were identified as potentially relevant. The title and abstract of the articles were reviewed. Seventy-five articles that fit the inclusion criteria were included. The selected articles were further reviewed by two researchers (RSR and DA) for their reliability. In case of any disagreement, consultation from a third reviewer (SVS) was employed. Among the 75 articles, 33 articles were excluded.
Study results
A total of 42 articles were selected based on the reviewer’s decision.[4],[6],[7],[8],[9],[11],[12],[17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34],[35],[36],[37],[38],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48],[49],[50],[51]
The selected articles included original research articles that used different biomarkers as a tool to elicit different genes which played an important role in tumorigenesis, metastasis, and radio and chemoresistance. The list of the selected articles is given in [Table 2]. A total of 15 biomarkers were identified in these studies among which the most commonly expressed markers were CD44, ALDH, and CD133. Twenty-five studies investigated CD44, while ten studies analyzed ALDH and six studies CD133. Seven studies combined the investigations on CD44 and ALDH, whereas three studies demonstrated the combined role of CD44 and CD133. Eight articles (Lee et al., Upadhyay et al., Shiina et al., Habu et al., Huang et al., Qiao et al., Koo et al and Tsai et al.) suggested the role of OCT 4, NANOG, and SOX2 as CSC markers in therapy resistance (both radio and chemotherapy) and reported that targeting these markers would lead to the inhibition of HNSCC. [Table 3] shows the most common CSC markers involved in tumorigenesis, metastasis, and therapy resistance.[24],[32],[33],[36],[39],[40],[45],[49] [Table 4] shows the difference between NSCs and CSCs.[52],[53]
| Table 2: Summary of selected articles Click here to view |
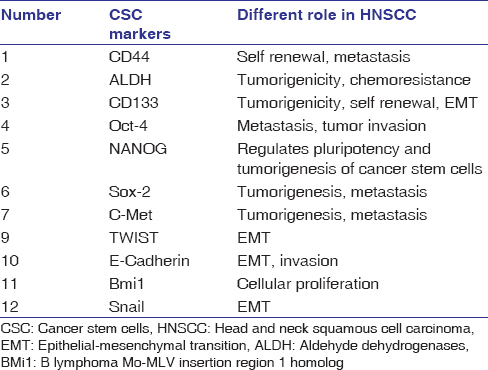 |
Table 3: List of most common cancer stem cell markers[1],[5] Click here to view |
| Table 4: Comparison between normal stem cells and cancer stem cells[52],[53] Click here to view |
The risk of bias of the original studies included is shown in [Figure 2]. Only one study was of high risk of bias.[51]
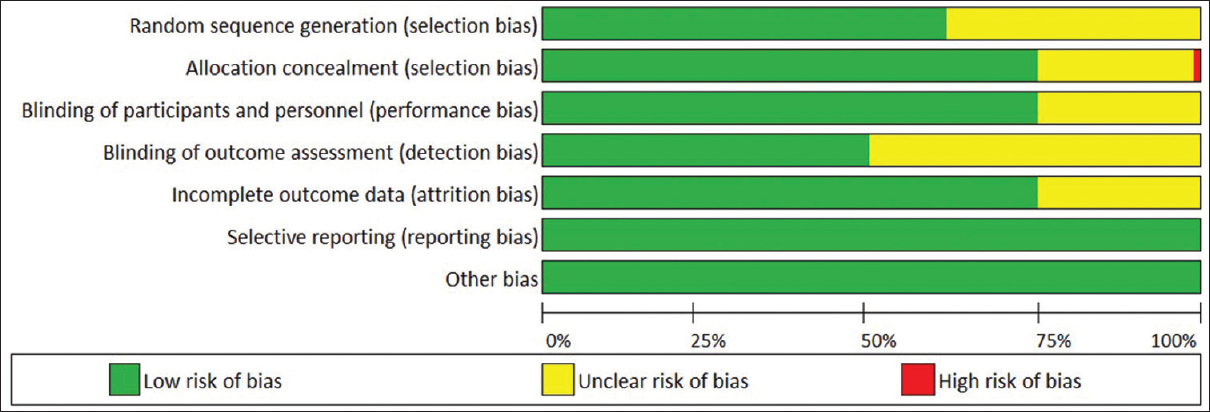 |
Figure 2: Assessment of risk of bias Click here to view |
A number of studies concluded that CSCs are virtually resistant to radiotherapy and chemotherapy through different mechanisms and lead to tumor relapse after therapy. [Table 5] enlists the causes for radioresistence and chemoresistance.[15],[54],[55],[56]
| Table 5: Causes of radioresistance and chemoresistance of cancer stem cells[15],[54],[55],[56] Click here to view |
Discussion
HNSCC is a common malignancy, being the eighth and thirteenth most common malignancy in the world for males and females, respectively.[1] Despite advancement in treatments, late-stage diagnosis results in poor prognosis and recurrence with metastases to locoregional lymph nodes. CSCs constitute a pool of self-sustaining cells with the ability to cause the heterogeneous lineages of cancer cells that compound the tumor. The term CSCs do not suggest their origin but the functional properties of the cells. CSCs possess significant resistance to current treatment modalities, such as chemotherapy and radiotherapy because of their abilities of self-renewal and regeneration.[1] For the establishment of prognostic biomarkers and target-specific drugs, recognition of accurate CSCs marker is necessitated.
CSCs show a tendency to be radio and chemoresistant; they also remain inactive for long periods of time and can evade conventional therapy. Even after the completion of treatment, these cells retain the capacity to become active and proliferate, tending to the establishment of distant metastasis and local recurrences. Conventional therapy is successful in scattering or debulking the tumor; chemotherapeutic drugs have shown a high rate of success [Figure 3]. It is suggested that slow-growing CSCs attempt to escape from conventional therapies, but in the passage of time, these cells are activated and regenerate tumors relatively with high recurrence rates.[1],[8],[9] Numerous studies have been done on CSCs, but none have identified an accurate marker or a panel of markers that can predict tumorigenesis, metastasis, and treatment resistance. To date, the most common modality in identifying CSCs in head and neck cancer relies on the expression of membrane cell surface antigens present in stem like cells [Figure 4].
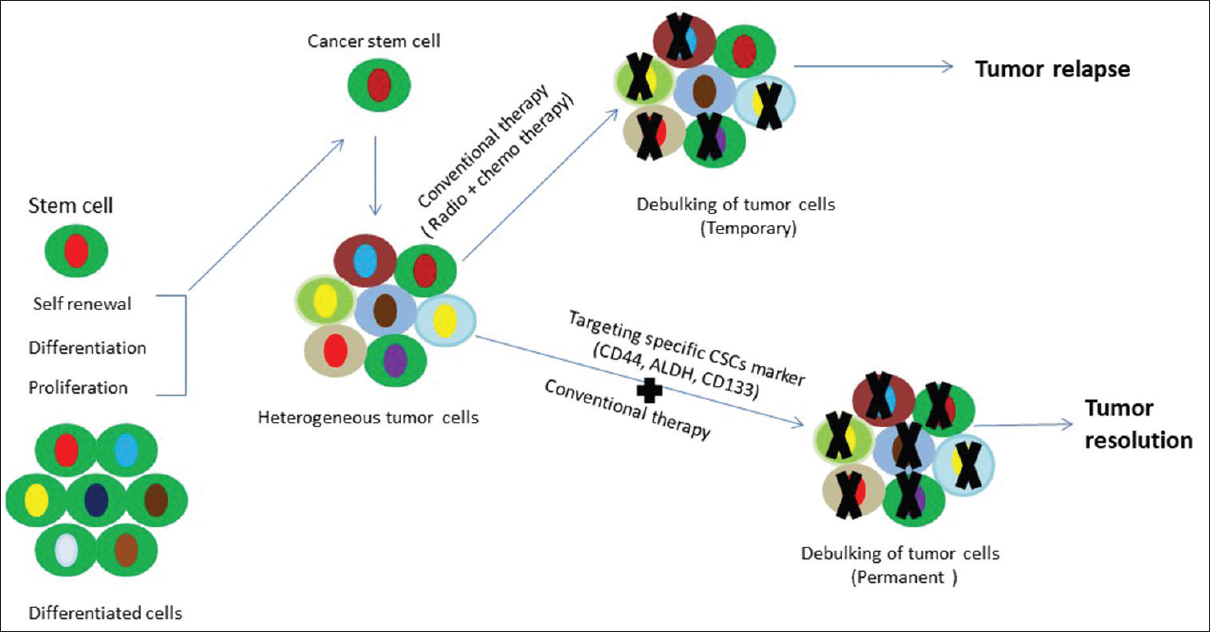 |
Figure 3: Schematic diagram showing conventional therapy and targeted therapy Click here to view |
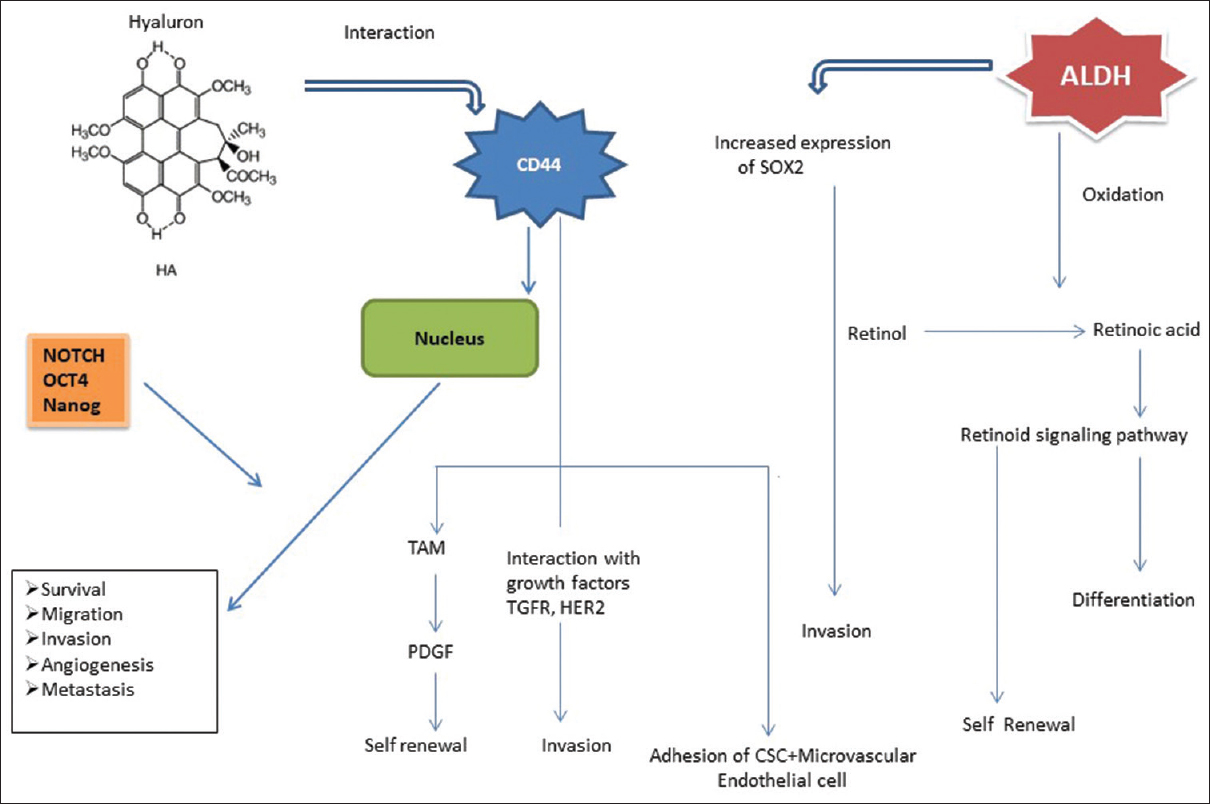 |
Figure 4: Role of stem cell proteins in cancer Click here to view |
CD44 is a well-known marker for CSC identification, and belongs to a large cell surface glycoprotein. It is thought to be involved in tumor progression and metastasis through its role as a regulator of growth, survival, differentiation, and migration. Many researchers have shown that subpopulations of CD44 containing cells which are emerging from both primary tissues and cell lines have higher potential for tumor sphere formation, differentiation, proliferation, migration, invasion, and resistance to chemotherapeutics.[11] The frequency of CD44-positive cells correlates with tumorigenesis, aggressive tumors, and higher rates of recurrence following radiotherapy. Higher CD44 expression is associated with poor prognosis and recurrence. CD44 v3 immunoexpression and CD44 v3+/CD24- immuno-phenotypes could give prognostic information associated with unfavorable clinical outcomes in the case of HNSCC.[7],[11],[23],[25],[34],[44] Mohanta et al. concluded that CD44 and CD147 together improve the prognostic efficacy of tumor differentiation, imparting the properties of increased self-renewal, migration, and invasion. A study conducted by Mannelli et al. showed CD44-positive cells with highest clonogenic capacity. Okamoto et al. found that HNSCC-CD44+ cells showed high expression levels of chemo-resistant genes (ABCB1, ABCG2, CYP2C8, and TERT) and indicated that CD44-positive cells were more resistant to chemotherapeutic agents compared to CD44-negative cells. Researchers found that the high expression of CD44 was correlated with a greater tendency for locoregional or distant metastasis and resistance to radio/chemotherapy. Grau et al. suggested that CD44 can interplay with estimated glomerular filtration rate which activates C-MET, focal adhesion kinase, and AKT signalling pathways that result in upregulation of reduced glutathione which in turn might lead to increase in radio-resistance.[47],[51],[57],[58]
ALDH1 is a member of the ALDH family of cytosolic isoenzymes, which are highly expressed in many NSCs and CSCs. The penta-span transmembrane glycoprotein has been identified as an evident CSC marker in majority of carcinomas such as skin, liver, brain, lungs, prostate, and colorectal cancers. This glycoprotein has the ability to induce spheroid formation, self-renewal, tumor formation, increased invasion capabilities, and resistance to chemotherapeutic treatment in HNSCC cell lines and primary tissue samples.
Kurth et al. found that inhibition of ALDH1A3-positive HNSCC cells might improve therapeutic response to radiotherapy as these cells may contribute to tumor relapse after irradiation. According to Chen et al., higher colony-forming ability was seen in cells with ALDH expression. There is a significant overlap in the ALDH and CD44 populations, with 50.6%–74.4% of ALDH1 cells expressing CD44. Huang et al. showed that ALDH, CD44, OCT4, and Sox2 are closely related in tongue squamous cell carcinoma and the expression of Sox2 can be used as a prognostic indicator. In contrast, a study by Leinung et al. suggested that neither ALDH nor CD44, alone or combined, was sufficient to determine the CSC population in HNSCC, but ALDH was shown to be a possible prognostic marker for poor survival. Chinn et al. concluded that CD44 high/ALDH-positive cells compared to CD44low/ALDH-negative cells have the capacity of tumorigenesis and greater rate of tumor growth.
Liu et al. conducted a study to evaluate the expression of ALDH1 and CD133 in oral lichen planus and OSCC. They found clinically significant value for ALDH positivity (48%) who developed oral cancer (P < 0.001). Similarly, 59.4% of patients having CD133 positivity developed oral cancer (P < 0.001). A multivariate analysis revealed that ALDH1 and CD133 expression was associated with 4.17-fold and 2.86-fold increased risk of oral cancer, respectively, and they concluded these markers can be considered as predictors to identify high risk of developing oral cancer.[26] Habu et al. evaluated the expression of Oct3/4 and NANOG in HNSCC cell lines. Oct3/4 showed a sensitivity of 82.0% and 61.5%, respectively. The author concluded that Oct3/4 and NANOG could represent probable CSC markers in HNSCC, and Oct3/4 could be considered as a potential predictor for distant nodal metastasis.[36]
CD133, another important stem cell marker, was found to be associated with either CD44 or ALDH. Lee et al. demonstrated that ectopic overexpression of CD133 significantly promotes properties of stemness in KB cell lines. Furthermore, CD133 promotes chemoresistance by arresting transition of the cell cycle and reducing apoptosis, which results in inhibition of tumor growth in fluorouracil- or cisplatin-injected mouse tumor model. They also reported that elevated levels of CD133 may lead to HNSCC chemo-resistance through increased stemness and cell cycle arrest. Many researchers have stated that carcinoma cell lines show enhanced clonogenicity in CD133-positive cells that are responsible for an EMT phenotype, tumor sphere formation, self-renewal, proliferation, tumorigenicity, and multilinear differentiation.[49],[59]
The present review also highlights the significant role of CSCs marker expression in HNSCC radiosensitivity. Radioresistant tumors show upregulation of stem cell markers such as CD44, ALDH, CD133, Oct-4, Sox2, NANOG, and BMI1, which are indicative of stemness and self-renewal. Ghuwalewala et al. concluded that CD44 high-CD24 low cell population displays increased CSC and EMT property.[44] In their study, cell lines of OSCC showed increased expression of Sox2, NANOG, and Oct-4. NANOG has been shown to be a therapeutic target controlling CSC self-renewal in HNSCC. Overexpression of Oct-4 might promote tumor-initiating properties in OSCC by mediating EMT.
Shiina et al. found that NANOG, Oct4, Sox2, and KLF-4 were upregulated in the CD44 v3 high and ALDH1 high cell population isolated from HNSCC and by adding 200 k Da-HA, it significantly decreased the ability of cisplatin to induce tumor cell death. This suggested both a decrease in tumor cell death and an increase in tumor cell survival, leading to the enhancement of chemoresistance. CSCs exhibit chemoresistance related to the ABC transporter expressed in these cells.[40] Notch is a signaling pathway which plays a role in both CSC maintenance and chemo-resistance. The Notch pathway is involved in the processes of tumor progression and metastases including tumor initiation, as well as self-renewal of CSCs.[4],[39],[45],[60] Upadhyay et al. pointed out that Notch activation is governed by gamma secretase inhibitor, or shRNA-mediated knockdown of Notch could lead to decreased capacity of spheroid formation, transformation, survival, and migration of HNSCC cells and concluded that targeting Notch signalling pathway may lead to better therapeutic outcome in tongue cancer.[39],[44],[45] Another major pathway is WNT/beta signaling pathway which maintains the self-renewal property of NSC and CSCs. The mechanism of chemoresistance through this pathway is still not completely understood, and varies among cell lines and tumor types. One potential mechanism is through the upregulation of ABC (ATP-binding cassette) pumps.[42],[48]
In addition to CSC biomarkers, microenvironmental factors, such as niche-specific properties, also represent potential therapeutic targets to allow the eradication of HNSCC cells. A niche is a microenvironment that supports CSC survival and growth, and niches may also represent potential therapeutic targets that need further research in HNSCC. Recent evidence suggests that HNSCC CSCs reside in perivascular niches which can represent a potential target, and therapeutic strategies exploiting the mutual dependence of CSCs and endothelial cells can reduce the rate of metastasis and recurrence in HNSCC.[10]
Few researchers have discussed differences in the biological behavior of NSCs and cancer cell. Genomic integrity is characteristic of NSCs, whereas in some tumorigenic cells, either loss of genomic integrity or scarcity of it makes them different from NSCs. Cancer cells have the capacity to access and maintain extragenetic mutations. In NSCs, the acquisition of differentiation is generally associated with loss of self-renewal capacity and overall reduction in cell proliferation. Tissue-specific stem cells give rise to limited number of differentiated cell types that are generally specific to one type of tissue. Interconversion among tumorigenic cancer cells is a significant mechanism which explains how these cells can become phenotypically heterogeneous and resist therapy.[60],[61],[62] Self-renewal and slow cycling are characteristics features shared by both NSCs in adult somatic tissues and CSCs.
Self-sufficiency for growth signals, insensitivity to antigrowth signals, evasion of apoptosis, limitless ability to replicate, sustained angiogenesis, tissue invasion, and metastasis make NSC different from CSCs. Identification and a better understanding of reliable molecular markers are needed to characterize CSCs in HNSCC. CSC markers themselves can serve as potential targets for anticancer therapy. Targeting CSC-specific markers and their molecular pathways may help in developing novel CSC diagnostics and therapeutic approaches. Along with conventional chemotherapy and radiotherapy, targeted therapy against the stem cell markers identified is an interesting prospect to research. Few limitations of this review were that original research articles published in language other than English were not considered. The selected articles were a combination of both in vivo and in vitro studies, and difference in expression of markers based on in vivo and in vitro issue is debatable.
Conclusion
Causes of failure of conventional therapy in HNSCC is a critical factor for local recurrence and distant metastasis. The research provides a new conceptual planning for anti-cancer therapy by identifying a sub-population of cells that possesses high tumorigenic potential. Currently, there is no single biomarker to define the CSC population accurately for HNSCC. A promising target panel of CSC markers identified through this systematic review were CD44, ALDH, and CD133, which were responsible for tumorigenesis, self-renewal, and therapy resistance, whereas NANOG, SOX-2, and OCT-4 were involved in metastasis and invasion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. |
Chuthapisith S. Cancer stem cells and chemoresistance. In: Shostak PS, editor. Cancer Stem Cells Theories and Practice. InTech; 2011. Available from: http://www. intechopen.com/books/cancer-stem-cells-theories-and-practice/ cancer-stem-cells-and-chemoresistance. [Last accessed on 11 Apr 2019].
 |
| 16. | |
| 17. | |
| 18. | |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. | |
| 25. |
Tan KM. The Expression of CD44s in Squamous Cell Carcinoma of the Oral Tongue and Association with Clinicopathological Factors and Survival Outcomes. Master’s Thesis. Singapore: National University of Singapore; 2012. Available from: http://scholarbank.nus.edu.sg/handle/10635/36363. [Last accessed on 14 Apr 2019].
 |
| 26. | |
| 27. | |
| 28. | |
| 29. | |
| 30. | |
| 31. | |
| 32. | |
| 33. | |
| 34. | |
| 35. | |
| 36. | |
| 37. | |
| 38. | |
| 39. | |
| 40. | |
| 41. | |
| 42. | |
| 43. | |
| 44. | |
| 45. | |
| 46. | |
| 47. | |
| 48. | |
| 49. | |
| 50. | |
| 51. | |
| 52. | |
| 53. | |
| 54. | |
| 55. | |
| 56. | |
| 57. |
Mannelli G, Magnelli L, Deganello A, Busoni M, Meccariello G, Parrinello G, et al. Detection of putative stem cell markers, CD44/CD133, in primary and lymph node metastases in head and neck squamous cell carcinomas. A preliminary immunohistochemical and in vitro study. Clin Otolaryngol 2015;40:312-20.
 |
| 58. | |
| 59. | |
| 60. | |
| 61. | |
| 62. |
Figures
[Figure 1], [Figure 2], [Figure 3], [Figure 4]
Tables
