Vrinda Singla1, Vipul Nautiyal2, Meenu Gupta2, Viney Kumar2, Shivani Mehra2, Mushtaq Ahmad2
1 Department of Radiation Oncology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
2 Department of Radiation Oncology, Cancer Research Institute, Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Dehradun, Uttarakhand, India
| Date of Submission | 17-Feb-2021 |
| Date of Decision | 03-Jun-2021 |
| Date of Acceptance | 27-Jul-2021 |
| Date of Web Publication | 27-Sep-2021 |
Correspondence Address:
Vipul Nautiyal
Department of Radiation Oncology, Cancer Research Institute, Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Doiwala, Dehradun – 248 016, Uttarakhand
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_5_21
Abstract
AIM: Clinical and dosimetric factors related to toxicity in terms of xerostomia in patients with head and neck squamous cell cancer (HNSCC) treated with intensity-modulated radiotherapy (IMRT).
MATERIALS AND METHODS: Patients older than 18 years, with the WHO Performance Status Score <2 with primary diagnosis of HNSCC Stage II, III, and IV who had undergone primary or postoperative radiotherapy (RT) treated by IMRT at the center, from November 2015 to November 2016 were included in the study. Patients were assessed by physical examination and questioned to score their quality of life for dryness (HNDR) and stickiness (HNSS) by EORTC-HN-35 (Hindi or English version) at baseline (before treatment), at 3, 6, and 12 months following treatment. The validation of EORTC-HN-35 for HNDR and HNSS in patients was handed.
RESULTS: Thirty patients were included in the study. The mean symptom score values for HNSS at baseline, 3, 6, and 12 months’ post-RT treatment were 17.8, 62.2, 64.4, and 20.8, respectively. Dryness and stickiness also increased over 3–6 months in follow-up but slightly relieved at 12 months, but it could not reach to baseline. In subgroup analysis, at baseline mean score of dryness of mouth in elderly patients (≥60 years) (P = 0.248), poor performance status (Eastern Cooperative Oncology Group 2) (P = 0.80) and patients with advanced stage (Stage III and IVA) (P = 0.185) was higher. Correlation of normal tissue complication probability for xerostomia with contralateral mean parotid gland showed insignificant linearity with shallow curve.
CONCLUSION: Patients remained symptomatic for xerostomia chiefly till 6 months’ postirradiation, but it was slightly relieved in 12 months but could not reach the baseline. Dosimetric sparing ofcontralateral parotid resulted in decreased probability of developing xerostomia.
Keywords: Head and neck cancer, intensity modulated radiotherapy, xerostomia
| How to cite this article: Singla V, Nautiyal V, Gupta M, Kumar V, Mehra S, Ahmad M. Study of dosimetry and clinical factors for assessment of xerostomia in head and neck squamous cell carcinoma treated by intensity-modulated radiotherapy: A prospective study. J Carcinog 2021;20:14 |
| How to cite this URL: Singla V, Nautiyal V, Gupta M, Kumar V, Mehra S, Ahmad M. Study of dosimetry and clinical factors for assessment of xerostomia in head and neck squamous cell carcinoma treated by intensity-modulated radiotherapy: A prospective study. J Carcinog [serial online] 2021 [cited 2021 Oct 24];20:14. Available from: https://carcinogenesis.com/text.asp?2021/20/1/14/326826 |
Introduction
Radiotherapy (RT) is an important therapeutic modality used either in adjuvant or primary setting for locally advanced setting of squamous cell carcinoma of head and neck (HNSCC). The most common late toxicity of radiation therapy is xerostomia where decreased salivary production leads to persistent dryness of mouth, sore throat, oral discomfort, tooth decay, dysgeusia, and alteration in swallowing leading to nutritional diminution and decreased weight.[1] Several studies assessed the efficacy of intensity-modulated RT (IMRT) in parotid gland sparing and reducing the xerostomia.[2]
The QUANTEC study group defined guidelines to minimize the radiation dose to the parotid glands aiming to reduce xerostomia after radiation treatment. These dose constraints have been based on normal tissue complication probability (NTCP)-modeling studies describing univariate associations between dose received by the parotid glands and xerostomia.[3] NTCP model formulated by either univariate or multivariate logistic regression model helps in predicting the probability of xerostomia.
Beetz et al. conducted a study to develop multivariable NTCP models for patient-rated moderate-to-severe xerostomia and sticky saliva among head and neck cancer (HNC) patients treated with three-dimensional (3D) conformal RT.[4] In further study, they assessed the validity of these NTCP-models among patients treated with IMRT.[5]
We introduced NTCP values of parotid gland from their respective dose-volume histogram (DVH) statistics and performed a prospective study in patients of squamous cell carcinoma of the head and neck treated by radiation using IMRT technique with the aim to assess the clinical and dosimetric factors related to xerostomia.
Materials and Methods
A prospective observational-based study was carried out from November 2015 to November 2016. The study was approved by the Institutional Ethics Committee. Informed written consent was taken from all patients. Thirty patients with primary diagnosis of head and neck squamous cell cancer Stage II, III, and IV Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of ≤2 who had undergone primary or postoperative RT were treated by IMRT were included in the study. Exclusion criteria were patients with a history of co-existing malignancy, ECOG PS >2, psychiatric illness, and tissue diagnosis other than squamous cell carcinoma. All patients underwent dental prophylaxis and dietary counseling before radiation treatment.
The demographic and tumor characteristics of these two study populations are listed in [Table 1].
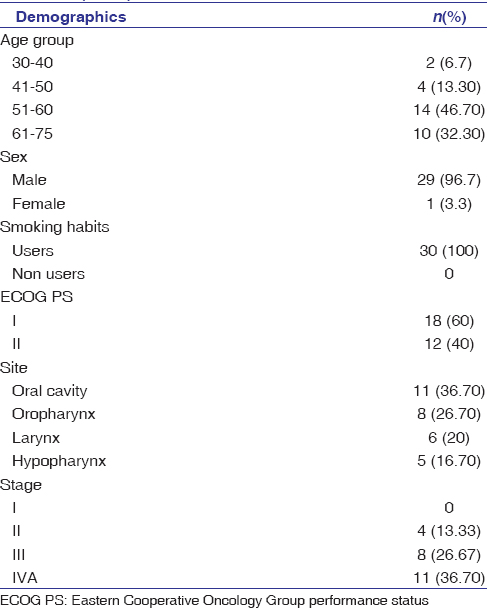 |
Table 1: Demographic profile of the patients at baseline (n=30) Click here to view |
Treatment planning
Patients planned for RT treatment underwent a contrast-enhanced planning computed tomography (CT) scan. The target volumes (gross tumor volume [GTV], clinical target volume [CTV], and planning target volume [PTV]) and normal tissues were delineated as per International Commission on Radiation Units (62, 83) on the CT images. The GTV was contoured as all known gross disease determined from CT scan, clinical information, and endoscopic findings.
A total dose of 60–66 Gy/30–33 number at 2–2.2 Gy/fraction over 6–7 weeks by IMRT technique with or without concurrent cisplatin (35 mg/m2) administered weekly or 3 weekly cisplatin (100 mg/m2). All the patients were treated with 5–7 field IMRT technique. The high risk CTV for postoperative patients included the primary tumor bed (based on preoperative imaging, physical examination, and operative findings) plus regions of grossly involved lymphadenopathy. The high risk clinical target volume for radical RT (CTV-66–70) of primary included the GTV + region of potential microscopic disease. CTVs were constructed with margin of 1–1.5 cm (minimum ~5 mm) around the GTV. Low risk CTV (CTV-54) included the drainage areas in the neck which may harbor subclinical disease.
Delineation of organs at risk including the brainstem, parotid glands, submandibular glands, optic nerves and chiasm, lens, spinal cord, and mandible was done.
DVH was generated for all critical normal structures and the unspecified tissues and dose limits were assessed from DVH of 3D volumes. Dose constraint for planning risk volume of spinal cord was 45 Gy. For salivary glands dose constraints were defined as either the mean dose to either parotid <26 Gy or at least 50% of either parotid received <30 Gy or at least 20cc of the combined volume of both parotids should not be >20 Gy. Attempt to save either of the parotids were made without compromising the PTVs.
During radiation therapy, patients were seen at least once every week in follow-up clinic. Subjective recording of treatment toxicity (according to the Radiation Therapy Oncology Group [RTOG] criteria) was done weekly. All patients were followed up at regular 3 months once the treatment was completed.
Subjective evaluation of patient’s perception of dry mouth and stickiness was done by EORTCQLQ HN35 manual version 3.0 at baseline and at 3, 6, and 12 months after completion of treatment. Patients were apprised about the questionnaire, due permission sought, and were advised to fill it independently. Question numbers 11 and 12 were specifically pertaining to dryness and stickiness of the mouth. For each parameter studied, for instance dryness of mouth in this study, a formula was used for calculating symptom score. Calculation of raw scores was done by (RSs); RS = (I1+ I2+ I3+… +In)/n. Symptom scales (SSs) scoring was done using the formula: SS = ([RS − 1]/Range) ×100 and range from 1 to 4. The scores were measured between 0 and 100 scales. A higher score on SSs denoted a low quality of life (QOL).
Statistical analysis
Statistical analysis was conducted utilizing SPSS Version 20 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp) and electronic spreadsheets (MS Excel). Interpretation and analysis of obtained results were carried out using appropriate tests. Qualitative data were expressed in terms of frequency/percentage. Quantitative data were expressed in terms of mean ± standard deviation repeated measure – ANOVA was used to compare the QOL score at different time intervals. NTCP models for patient-rated xerostomia and sticky saliva were developed using a multivariable logistic regression analysis with an extended bootstrapping technique.[4]
Adjusted odd’s ratios and 95% confidence intervals were calculated for the selected variables in the model. For each patient, observed and predictive values were calculated for each set of prognostic variables based on the regression coefficients according to the formula:
NTCP = (1 + e − s) − 1, where S = −1.443+ (mean dose contralateral parotid gland × 0.0470) + (baseline xerostomia score × 0.720)
Baseline and 3 months xerostomia scores were used for predicted and observed NTCP respectively.
There are various different validation models[6],[7] and Nagelkerke’s R2 explained the variation in his tools.[8] In addition, model performance was evaluated using measures for discriminative ability, including the area under the receiver operating curve.[9],[10] Model performance was further quantified in terms of calibration, i.e., the agreement between predicted and observed outcome in the dataset, while the Hosmer–Lemeshow “goodness-of-fit” test was used frequently in risk prediction models. The test assesses whether or not the observed event rates match expected event rates in subgroups of the model population.[11]
Results
The median follow-up of the present study was 18 months. The median age was 56 years. Majority of patients in the study were males, i.e., 29 (96.7%) and 1 (3.3%) was female. All patients were smokers.
Dosimetric parameters, i.e., PTV coverage and mean dose to contralateral (C/L) and ipsilateral (I/L) parotid glands were studied using DVH. Mean D95 for PTV was 97%. The mean dose received by the I/L and C/L parotid glands were 37.5 and 25.9 Gy, respectively.
At the end of 6 weeks of treatment, 8 patients developed RTOG Grade 2 acute mucositis and 22 patients developed RTOG Grade 3 acute mucositis. Five (16.6%) and 17 (56.7%) patients developed Grade 1, Grade 2 dysphagia respectively while 8 (26.6%) patients developed Grade 3 dysphagia during external beam radiation therapy (EBRT) treatment. Fourteen patients were put on nasogastric tube feeding during the treatment for nutritional support.
QOL for dryness (HNDR) and stickiness (HNSS) were assessed in 30 patients by using EORTC-HN-35 (Hindi version or English version) at baseline (before starting the RT), at 3, 6, and 12 months following treatment.[Figure 1] depicted the mean symptom score values for HNDR at baseline, 3, 6, and 12 months post-RT were 21.1, 44.4, 56.7, and 33.3, respectively. The mean symptom score values for HNSS at baseline, 3, 6, and 12 months’ post-RT were 17.8, 62.2, 64.4, and 51.1, respectively. Dryness and stickiness also increased over 3–6 months in follow-up but it has slightly improved at 12 months but it could not reach the baseline.
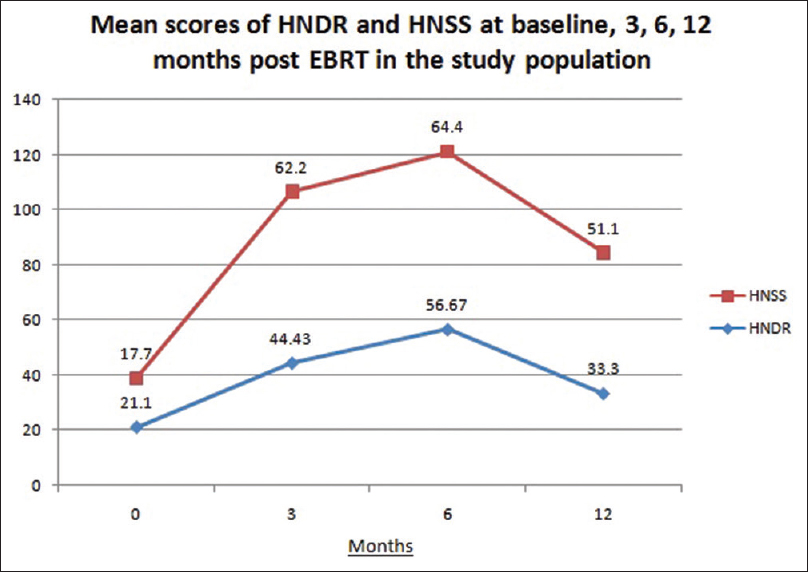 |
Figure 1: Mean scores of quality of life for dryness and stickiness at baseline, 3, 6, and 12 months postexternal beam radiation therapy Click here to view |
In subgroup analysis, at baseline mean score of dryness of mouth in elderly patients (≥60 years) was higher (HNDR 0 = 27.3) compared to patients aged <60 (HNDR 0 = 17.3) (P = 0.248). In patients with poor performance status (ECOG PS 2) at presentation mean HNDR 0 was 24.02 while in patients with ECOG PS 0 or 1 mean HNDR 0 score was 16.09 (P = 0.80). Patients with advanced stage disease (Stage III and IVA) also had baseline mean dryness score higher (HNDR 0 = 23.05) compared to early stage diseases (Stage II) (HNDR 0 = 8.33) (P = 0.185). We could not found any difference in baseline HNSS0 in reference to age, performance status and stage of the disease.
In our study, linear correlation [Figure 2] between contralateral parotid dose and NTCP for xerostomia at presentation and 3 months was seen but with shallow curves (insignificant P = 0.380). Correlation between predicted NTCP and observed NTCP for xerostomia was observed to be linear with significance (P < 0.001). The calibration slope of 1.0 [Figure 3], indicated a good agreement between observed and predicted NTCP values.
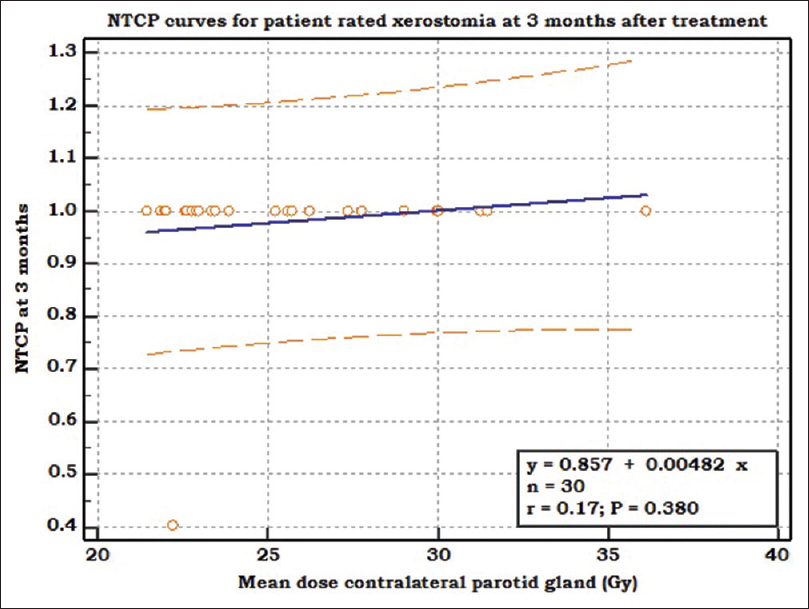 |
Figure 2: NTCP curve depicting linear relationship (blue line) between mean dose to contralateral parotid gland (X-axis) and NTCP at 3 months (Y-axis). NTCP: Normal tissue complication probability Click here to view |
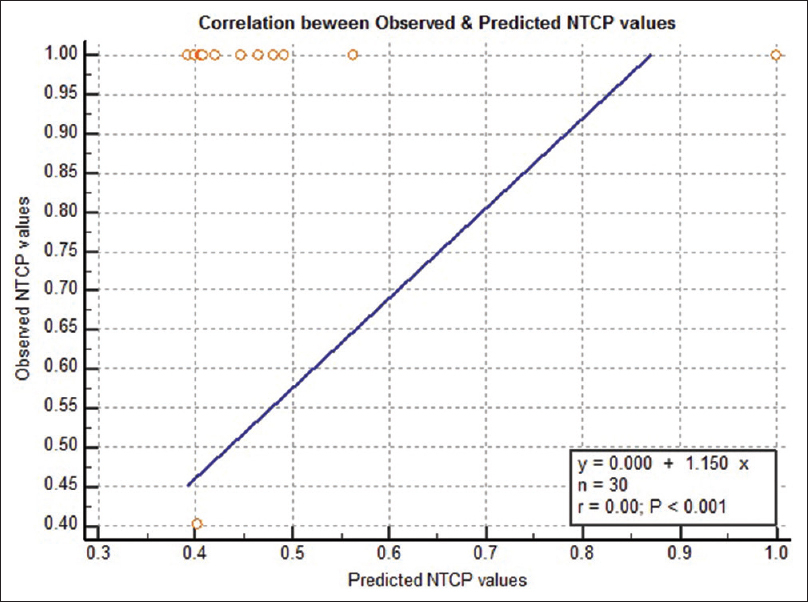 |
Figure 3: Graph depicting linear correlations (blue line) between predicted NTCP (X-axis) and observed NTCP (Y-axis). NTCP: Normal tissue complication probability Click here to view |
At the end of 12 months’ follow-up, out of 21 alive patients, 16 were without disease, 4 had loco-regional failure and 1 developed distant metastasis. 4 patients died and 5 were lost to follow-up.
Discussion
The treatment of HNSCC has undergone a paradigm shift over the past two decades, with advances in surgery, radiation therapy, and chemotherapy leading to improved locoregional control, survival, and QOL. The outcomes of these treatment modalities have shifted the focus of curative efforts from radical ablation to preservation and restoration of function.[12]
RT plays a crucial role in combined-modality therapies for patients with locally advanced SCCHN. Due to its advantages in terms of greater dose conformity for complex tumor targets and better protection of adjacent critical normal structures, IMRT is increasingly widely used.[13]
In our study, we also observed the correlation of clinical factors with baseline HNDR score and observed that elderly patients, patients with poorer ECOG PS and higher T stage had a higher dryness of mouth score although results were not significant. Beetz et al. in their study concluded that patient-rated xerostomia is a complex endpoint, which can be influenced by several factors either related to dose distributions to major and minor salivary glands, or by other factors such as baseline xerostomia, age, and medication.[14]
We spared the contralateral parotid gland, i.e., reduced the mean parotid dose to below 26 Gy.
For parotid glands, constraints were used based on the report published by Eisbruch et al. who defined the mean threshold dose (mean dose that reduces the saliva flow rate to <25% of pretreatment level) to be 24 and 26 Gy respectively for unstimulated and stimulated parotid saliva flow respectively for contralateral parotid gland.[15] In our study, we looked at xerostomia which is a prevalent complication among the HNC patients treated with EBRT. We observed that the symptom of dryness and sticky saliva had worsened at 3, 6 months compared to baseline and then comparatively recovered in 12 months. The mean symptom score of sticky saliva also progressively increased at 3 and 6 months after EBRT, but it showed slight recovery in 12 months. Significant increase in dryness and sticky saliva score was also seen at 3 and 6 months as compared to baseline and the mean score remained similar at 6 months when compared with score at 3 months. In a study conducted by Pierre Graff et al., they found that the mean scores for dry mouth at 6 months after completion of EBRT were 69.91 which recovered significantly at 12 months to 57.2, while the mean scores for sticky saliva at 6 months after completion of EBRT were 58.53 which recovered significantly at 12 months to 47.12. Our study was small and had a short follow-up and our patients continued to be symptomatic on account of dry mouth and sticky saliva. Symptomatic relief was expected at 12 months.[16]
Moderate-to-severe xerostomia was seen in 24 patients (80%) at 3 months and in 19 patients (63.3%) at 6 months which showed some recovery at the end of 6 months although not statistically significant. A study done by Lee et al. showed that significant parotid function recovery occurred at the end of 12 months.[3]
This was in accordance with the study done by Lee et al. in which they saw that for the 3-month time point, the mean dose to the contralateral parotid gland was the most important variable determining acute xerostomia.[3]
We found linear correlation between contralateral parotid dose and NTCP for xerostomia at presentation and 3 months. This observation is in line with the available literature which says that although parotid gland dysfunction plays an important role in the development of patient-rated xerostomia, it is not the only prognostic factor. Beetz et al. recently showed that age and baseline xerostomia were independent prognostic factors for patient-rated xerostomia, in addition to the mean dose to the parotid glands.[17] Significant linear correlation between predicted NTCP and observed NTCP (P < 0.001) clearly indicates that the dosimetric parameters and the baseline xerostomia score taken into account for keeping in check the severity and probability of xerostomia are of value.
Conclusion
Our study suggested that a positive correlation existed between the dosimetric as well as clinical factors in the assessment of xerostomia in patients of HNSCC treated with IMRT technique. These parameters can be used to optimize the patient treatment to minimize toxicity. However, our study had some shortcomings. The sample size in our study was small. To concur a meaningful conclusion, we need to accrue more patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
| 1. |
Vassilis Kouloulias, Stella Thalassinou, Kalliopi Platoni. The Treatment Outcome and Radiation-Induced Toxicity for Patients with Head and Neck Carcinoma in the IMRT Era: A Systematic Review with Dosimetric and Clinical Parameters. BioMed Research International, vol. 2013, Article ID 401261, 12 pages, 2013. doi: 10.1155/2013/401261.
 |
| 2. | |
| 3. | |
| 4. | |
| 5. |
Beetz I, Schilstra C, van Luijk P, Christianen ME, Doornaert P, Bijl HP, et al. External validation of three dimensional conformal radiotherapy based NTCP models for patient-rated xerostomia and sticky saliva among patients treated with intensity modulated radiotherapy. Radiother Oncol 2012;105:94-100.
 |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. |
Beetz I, Schilstra C, van der Schaaf A, van den Heuvel ER, Doornaert P, van Luijk P, et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: The role of dosimetric and clinical factors. Radiother Oncol 2012;105:101-6.
 |
| 15. | |
| 16. | |
| 17. |
Figures
[Figure 1], [Figure 2], [Figure 3]
Tables
[Table 1]
