Ji-Kang Jeong1, Hee-Kyung Chang2, Kun-Young Park1
1 Department of Food Science and Nutrition, Pusan National University, Busan 609-735, Korea
2 Department of Pathology, Kosin University Gospel Hospital, Busan 602-702, Korea
| Date of Submission | 09-Jan-2014 |
| Date of Acceptance | 20-Jun-2014 |
| Date of Web Publication | 29-Jul-2014 |
Correspondence Address:
Kun-Young Park
Department of Food Science and Nutrition, Pusan National University, Busan 609-735
Korea
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1477-3163.137699
Abstract
Backgrounds: Doenjang is traditional Korean fermented soybean paste and widely known for its various health benefits including anticancer effect. In this study, we manufactured doenjang with the grain-type meju using probiotic mixed starter cultures of Aspegillus oryzae, Bacillus subtilis-SKm, and Lactococcus lactis-GAm to improve the qualities and beneficial properties of doenjang. Materials and Methods: The inhibitory effects of the doenjang prepared with the grain-type meju using mixed starter cultures were investigated in azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced colon carcinogenesis mice model. AOM and DSS colon carcinogenesis was induced in female C57BL/6 mice, and doenjang was orally administered for 4 weeks. Body weight, colon length, and colon weight of mice were determined, and colonic tissues were histologically evaluated. The serum levels of proinflammatory cytokines as well as the expression of inflammation- and apoptosis-related genes in colonic tissue were also analyzed. Results: Administration of the doenjang using probiotic mixed starter cultures ameliorated the symptoms of colon cancer, and reduced the incidence of neoplasia, and reduced the levels of serum proinflammatory cytokines such as interleukin-6, and tumor necrosis factor-α and inducible nitric oxide synthase and cycloooxygenase-2 expression levels in colonic tissue. In addition, it increased Bax and reduced Bcl-2 expression levels and increased p21 and p53 expression in the colonic tissues. Conclusion: These findings indicate that the doenjang attenuated colon carcinogenesis induced by AOM and DSS by ameliorating the symptoms of colon cancer, reducing the occurrence of neoplasia, regulating proinflammatory cytokine levels, and controlling the expressions of inflammation- and apoptosis-related genes in the colonic tissue.
Keywords: Colon carcinogenesis, doenjang, inflammation, mixed starter
Introduction
Doenjang is a representative Korean traditional fermented soybean foods and well known to possess various health benefits including antimutagenic and anticancer effects. [1],[2] It has been reported that doenjang has higher functionality than other fermented soybean foods, such as Japanese miso, and actual soybean. [2] Doenjang has been traditionally manufactured from meju, which is a main ingredient and starter culture, made of cooked and crushed soybeans. [3],[4] Recently, doenjang has been commercially produced by several manufacturing companies, and the traditional method has had to be modified and adapted for mass production. In general, doenjang manufactured through the traditional method has superior taste and functionalities compared to commercially produced doenjang because various naturally occurring microorganisms are related to traditional meju and doenjang fermentation. However, problems regarding food safety and inconvenience of traditional doenjang have been consistently reported. Therefore, attempts have been made to develop doenjang manufacturing techniques that take advantage of the traditional method and increase food safety. [5],[6] One of these methods involves the use of effective and beneficial microorganisms as starter cultures.
Colorectal cancer (CRC) is a major health problem worldwide, and much evidence has verified that chronic inflammation is a key predisposing factor for the development of CRC. [7] Especially patients with inflammatory bowel disease such as ulcerative colitis are at high-risk for CRC. [8] Epidemiological studies have revealed an association between nutrition habit and the prevention of a certain type of cancer, including CRC. In addition, several functional foods and chemicals such as fermented soybean foods, curcumin, and epigallocatechin gallate have been reported to inhibit colon carcinogenesis in vitro and in vivo.[9],[10],[11]
Cycloooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) are the primary enzymes associated with colon carcinogenesis in the context of chronic inflammation. COX-2 expression is initiated by reactive oxygen species and tumor promoters, as well as stimulation by proinflammatory cytokines such as tumor necrosis factor-α (TNF-α). In addition, up-regulated COX-2 generates prostaglandins that can promote cell growth and angiogenesis. iNOS produces nitric oxide (NO), which stimulates COX-2 activity and increases the inactivation of the p53 tumor suppressor gene. [12],[13] Moreover, the lack of p53 activation results in the downregulation of cyclin-dependent kinase (Cdk) inhibitors such as p21 and proapoptotic genes such as Bax. [14]
In the previous study, we developed the grain type meju using probiotic mixed starter cultures to improve the qualities and functionalities of fermented soybean products such as doenjang, as well as meju itself. And we verified its improved qualities and functionalities especially antiinflammatory and anticancer effects using azoxymethane/dextran sulfate sodium (AOM/DSS) induced colitis associated colon cancer mice model. [15],[16] In the current investigation, we manufactured doenjang using this grain-type meju and attempted to demonstrate its health functionality using same mouse colon cancer model. We observed the general conditions of the mice and evaluated the serum levels of proinflammatory cytokines such as interleukin-6 (IL-6) and TNF-α. Histological analysis was also conducted through hematoxylin and eosin (H and E) staining, and inflammation- and apoptosis-related gene levels were also analyzed in the colonic tissues of mice.
Materials and Methods
Preparation of the starter cultures
Bacillus subtilis-SKm (KFCC11520P), and Lactococcus lactis-GAm (KFCC11510P) used in this study, were isolated from Korean traditional meju. These bacteria were selected as starters for manufacturing doenjang due to their higher enzyme activity, probiotic effect, and functionality than other isolates in our previous study. [15] B. subtilis-SKm (KFCC11520P), and L. lactis-GAm were inoculated nutrient broth and MRS broth, respectively, and incubated at 37°C for 24-48 h before use. Aspegillus oryzae was purchased from Chungmoo Fermentation (Ulsan, Korea) and maintained at − 20°C before use.
Doenjang preparation
To prepare doenjang, grain type meju was first manufactured. To prepare the meju, soybeans were washed and soaked in water at 20°C for 12 h, and steamed at 121°C for 30 min in an autoclave. The steamed soybeans were cooled to 45-50°C, and then inoculated with 0.2% (10 6 spores/g) A. oryzae and 10 6 cfu/g B. subtilis-SKm before being incubated at 30°C for 48 h. Then, the fermented soybeans were inoculated with 10 6 cfu/g L. lactis-GAm and incubated at 37°C for 24 h.
The prepared grain type meju, water, and salt were mixed at the ratio of 1:1.7:0.46 (wt: wt: wt), placed in a jar, and fermented at 37°C for 6 weeks. [15] Finally, doenjang using probiotic mixed starter cultures was prepared, and it was designated as A. oryzae, B. subtilis SK-m, and L. lactis-GAm inoculated doenjang (ABL-d). For a comparison with this doenjang in further studies, doenjang prepared with commercial grain type meju (CG-d) obtained from Alalee Food Co. (Koryung, Korea) was used. It is reported that doenjang made from this commercial meju exhibited strong anticancer and antimutagenic effect by our previous study. [1]
Azoxymethane and dextran sulfate sodium-induced colitis associated cancer model
Specific pathogen free, 7-week-old C57BL/6 mice (Central Lab Animal Inc., Seoul, Korea) were randomly allocated as eight mice in each group, housed under standard conditions (50-60% humidity, 12 h dark/12 h light cycle). Food and water freely supplied. Mice in sample treatment groups were given single intraperitoneal injection of AOM (10 mg/kg, AOM, Sigma Co., St. Louis, MO, USA). Starting 2 and 5 weeks after the injection, animal received 2.5% DSS in the drinking water for 7 days. Doenjang were orally administered as 2 g/kg dose weeks after the injection for 4 weeks. Normal group received 0.1 ml phosphate buffered saline (PBS) as a vehicle after the onset of the experiment for 4 weeks without AOM and DSS and control group were given single intraperitoneal injection of AOM and then, received 2.5% DSS in the drinking water after 2 and 5 weeks for 7 days. PBS was orally administered 2 weeks after the injection for 4 weeks. Total experimental period was 8 weeks. [17] The protocol used for this study was approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU; approval number PNU-2011-000408) of PNU (Busan, Korea).
Serum and tissue sample preparation
At the end of the experiment, blood samples from each animal were collected without anticoagulant treatment. Serum was separated from the blood by centrifugation at 3500 rpm for 10 min. Colons were taken directly from mice, rapidly measured their length and weight, and frozen in liquid nitrogen. The samples were stored at − 80°C until use.
Histological examination
At the end of the experimental period, the colons were removed and cleaned in saline to remove fecal residues. The colon tissues were fixed with 10% (v/v) buffered formalin, and embedded in paraffin. Four micrometer-thick slices from paraffin sections were stained with H and E prior to microscopic observation. Histological analysis was performed by pathologist who was unaware of the experimental protocol. A histological score was established in a scale of 0-4, where 0 = No signs of damage; 1 = Few and slight signs of damage, 2 = Mild signs of damage; 3 = Moderate signs of damage; 4 = Severe and heavy signs of damage. [18]
Serum proinflammatory cytokine analysis in serum
Concentration of the serum proinflammatory cytokines, IL-6, IL-12, TNF-α, and interferon-gamma (IFN-γ) were determined using a commercially available ELISA kit (ELISA MAX, Biolegend, San Diego, CA, USA).
Reverse transcription polymerase chain reaction
Gene expression was measured by reverse transcription polymerase chain reaction (PCR) in an ExiCycler (Bioneer, Daejeon, Korea). Briefly, total RNA was isolated from tissues in mice using Trizol reagent (Invitrogen, Carlsbad, CA, USA). One microgram of total RNA was used for first-strand cDNA synthesis using Superscript II reverse transcriptase (BD Bioscience, Palo Alto, CA, USA). Reverse transcription was performed at 30°C for 10 min, 42°C for 30 min, and 99°C for 5 min to inactivate the avian myeloblastosis virus RTXL. Amplification was performed in a master-cycler (Eppendorf, Hamburg, Germany) with denaturing at 94°C for 1 min, annealing at 54°C for 1 min, extension at 72°C for 30 s for 25 cycles and finally 72°C for 7 min. The amplified PCR products were run in 1.0% agarose gels and stained with ethidium bromide, and visualized under ultraviolet light. The intensities of the bands were estimated by densitometry (Multi Gauge V3.0 software, Fujifilm Life Science, Tokyo, Japan).
Statistical analysis
All results obtained were analyzed in time design using the general linear model procedure of the SAS System (SAS Institute, Cary, NC, USA). Data are expressed as mean ± standard deviation values. Means with different letters are significantly different (P < 0.05) by Duncan’s multiple range tests.
Results
General observations
[Table 1] showed that the changes of body weight during the experimental period. The weight gains of AOM and DSS receiving mice were slightly slower, however, all group gained weights and any noticeable differences in body weight of mice belonged to each group were not observed. Liver and spleen weights of AOM and DSS treated group were significantly heavier than normal group at the end of this study. Kidney weight of AOM and DSS treated group were also heavier than normal group [Table 2]. These all organ weights showed no noticeable changes in CG-d group compared to the control group. However, these organ weights, especially liver weight of ABL-d were significantly lighter than those of the control group (P < 0.05).
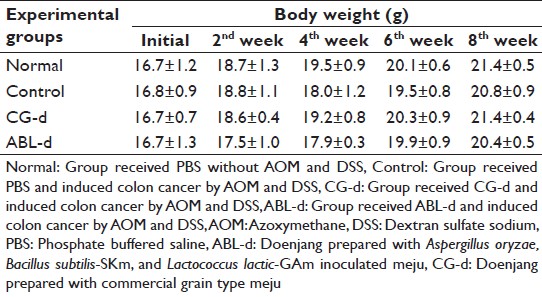 |
Table 1: Effects of doenjang treatment on the body weight of AOM and DSS-induced colon cancer mice during doenjang treatment Click here to view |
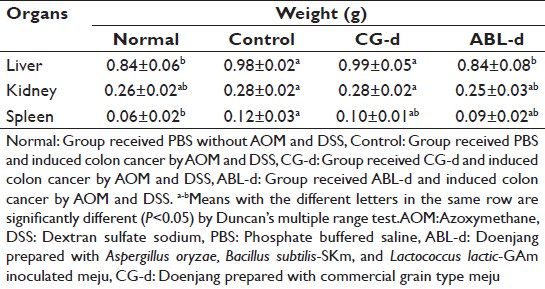 |
Table 2: Effects of doenjang treatment on the organ weight of AOM and DSS-induced colon cancer mice at the end of the experiment Click here to view |
The colon length of AOM and DSS treated control group was shortened compared to that of normal group, however, this phenomenon was not much severe unlike in the DSS-induced colitis mice model. [15] The colon weight of the control group increased compared with the normal group. These colon length and weight became similar to the normal group in doenjang treated group [Table 3]. The weight/length ratio of colon was significantly increased in AOM and DSS treated control group compared with the normal group. The increased colon weight/length ratio was recovered in doenjang treated group and significantly low in ABL-d treated group (P < 0.05).
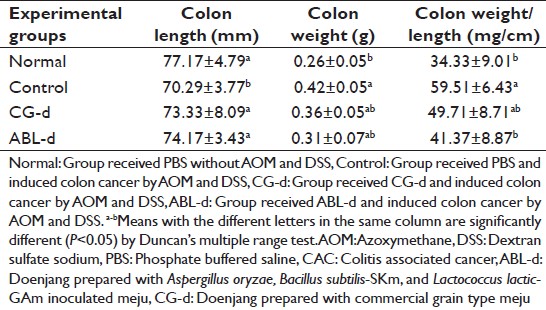 |
Table 3: Effects of doenjang treatment on colon length and colon weight of AOM and DSS-induced CAC mice Click here to view |
Histological observations
Macroscopically, nodular, and polypoid colonic tumors were observed in the middle and distal colon of all AOM and DSS treated mice. Microscopic view of the colon of mice treated with AOM and DSS and also those given doenjang are shown in [Figure 1]. AOM and DSS treated mice resulted in histological alteration of the colonic mucosa including inflammation with mucosal ulcer, infiltration of inflammatory cells into the lamina propria, and loss of crypts. And the polyps were exhibited high-grade of dysplasia, and they were mostly adenoma and adenocarcinoma. However, doenjang treatment decreased the loss of crypt in the colonic mucosa and relieved the inflammation of the colonic mucosa. In addition, the administration of CG-d and ABL-d appeared to induce the apoptosis of colon tumor cells.
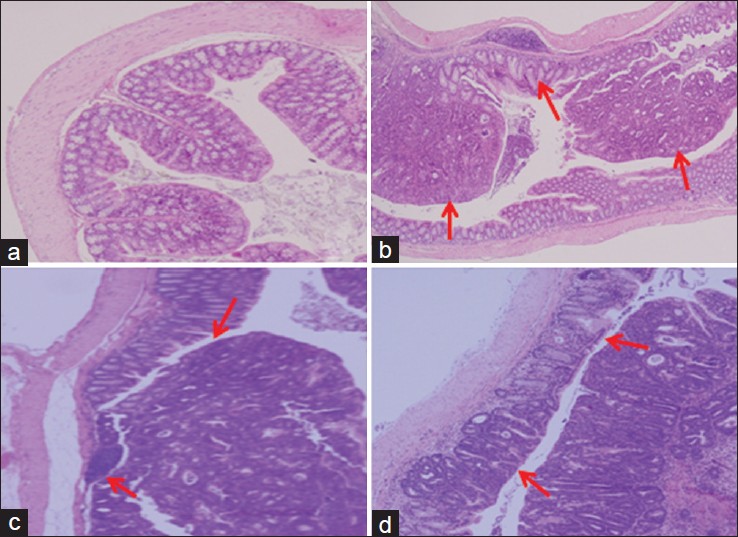 |
Figure 1: Histological images of the colonic mucosa of azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced colitis associated cancer mice. (a) Normal, group received phosphate buffered saline (PBS) without AOM and DSS; (b) Control, group received PBS and induced colon cancer by AOM and DSS; (c) CG – d, group received CG – d and induced colon cancer by AOM and DSS; (d) ABL – d, group received ABL – d and induced colon cancer by AOM and DSS Click here to view |
The number, size, and location of detectable tumors were macroscopically examined in the colon from the animals treated AOM and DSS. Mice treated with AOM and DSS had 100% incidence of colonic tumors, which were ranged from 1 to 3 mm in size. However, the multiplicity of tumors was lowered by the administration of doenjang. Especially, ABL-d significantly reduced total number of colonic neoplasia by 34.5% [Figure 2].
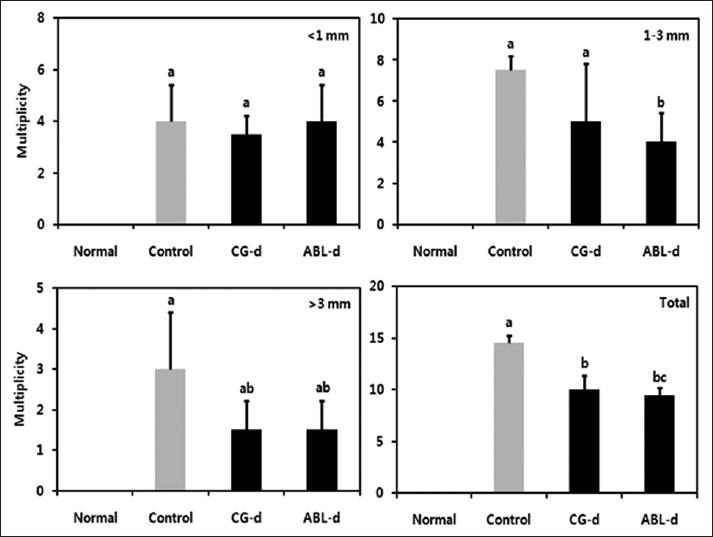 |
Figure 2: Effects of doenjang treatment on the incidence and size of colonic neoplasms. Normal, group received phosphate buffered saline (PBS) without azoxymethane (AOM) and dextran sulfate sodium (DSS); Control, group received PBS and induced colon cancer by AOM and DSS; CG – d, group received CG – d and induced colon cancer by AOM and DSS; ABL – d, group received ABL – d and induced colon cancer by AOM and DSS. a-cMeans with the different letters are significantly different ( P<0.05) by Duncan’s multiple range test Click here to view |
Proinflammatory cytokine levels
To investigate the role of proinflammatory cytokines such as IL-6, IL-12, TNF-α, and IFN-γ in colitis associated colon cancer, we analyzed the production of the cytokines in serum [Figure 3]. In the control group, IL-6, IL-12, TNF-α, and IFN-γ levels were significantly enhanced, and this contrasted with the normal group. However, these four cytokines levels were significantly diminished in doenjang treatment group. Especially, IL-6 and IFN-γ levels were significantly low in ABL-d group (P < 0.05).
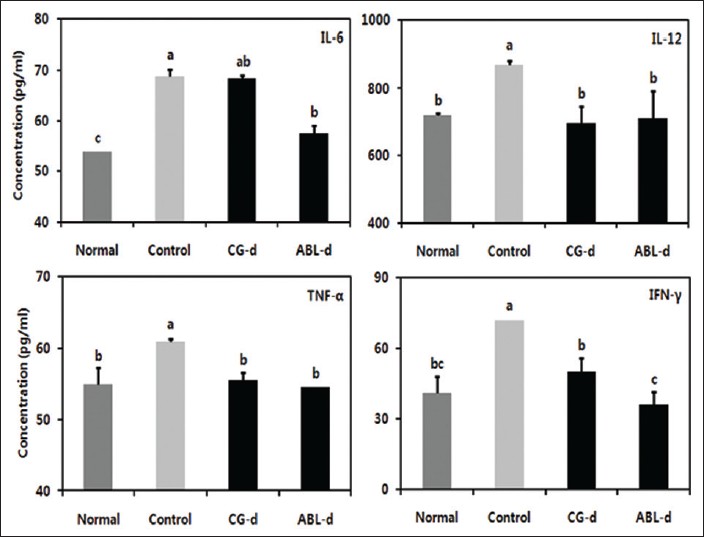 |
Figure 3: Effects of doenjang treatment on serum proinflammatory cytokines in azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced colitis associated cancer mice. Normal, group received phosphate buffered saline (PBS) without AOM and DSS; Control, group received PBS and induced colon cancer by AOM and DSS; CG-d, group received CG-d and induced colon cancer by AOM and DSS; ABL-d, group received ABL-d and induced colon cancer by AOM and DSS. a-cMeans with the different letters are significantly different ( P< 0.05) by Duncan’s multiple range test Click here to view |
Expression of inducible nitric oxide synthase and cycloooxygenase-2 in colon tissue
Many accumulating evidences indicate an important role of iNOS and COX-2 in colon cancer growth and progression. Therefore, the expressions of iNOS and COX-2 in colonic tissue were examined [Figure 4]. iNOS and COX-2 expressions significantly augmented in the control group compared to the normal group. On the contrary, the reduction of these two factors was significant in the mice administered with doenjang, particularly ABL-d.
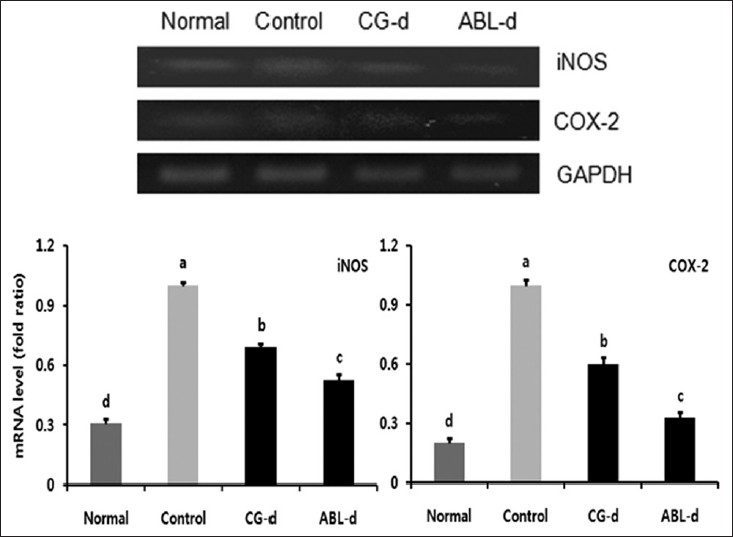 |
Figure 4: Ef fects of doenjang treatment on messenger RNA expressions of iNOS and COX-2 in the colon tissue of azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced colitis associated cancer mice. Normal, group received PBS without AOM and DSS; Control, group received PBS and induced colon cancer by AOM and DSS; CG-d, group received CG-d and induced colon cancer by AOM and DSS; ABL-d, group received ABL-d and induced colon cancer by AOM and DSS. a-dMeans with the different letters are significantly different ( P< 0.05) by Duncan’s multiple range test Click here to view |
Expression of Bax, Bcl-2, p53, and p21 in colon tissue
Bax and Bcl-2 are members of Bcl-2 family of apoptosis regulators. Over-expression of Bax has been shown to induce apoptosis, and Bcl-2 can block cell death in various cell systems. [19] In this study, pro-apoptotic Bax significantly decreased, and antiapoptotic Bcl-2 increased in DSS and AOM induced colitis associated colon cancer mice, and they showed a contrary tendency in doenjang, particularly ABL-d treated group [Figure 5].
 |
Figure 5: Effects of doenjang treatment on messenger RNA expressions of Bax and Bcl – 2 in the colon tissue of azoxymethane (AOM) and dextran sulfate sodium (DSS) – induced colitis associated cancer mice. Normal, group received phosphate buffered saline (PBS) without AOM and DSS; Control, group received PBS and induced colon cancer by AOM and DSS; CG – d, group received CG – d and induced colon cancer by AOM and DSS; ABL – d, group received ABL – d and induced colon cancer by AOM and DSS. a-dMeans with the different letters are significantly different ( P<0.05) by Duncan’s multiple range test Click here to view |
p53 is involved in the control of cell growth, tumor growth, cell cycle arrest, and apoptosis reflecting p21, Cdk inhibitor and/or Bcl-2 family expression. As shown in [Figure 6], p21 and p53 genes were diminished significantly by AOM and DSS treatment in animals. However, the expression of these genes were recovered in doenjang, especially ABL-d fed group.
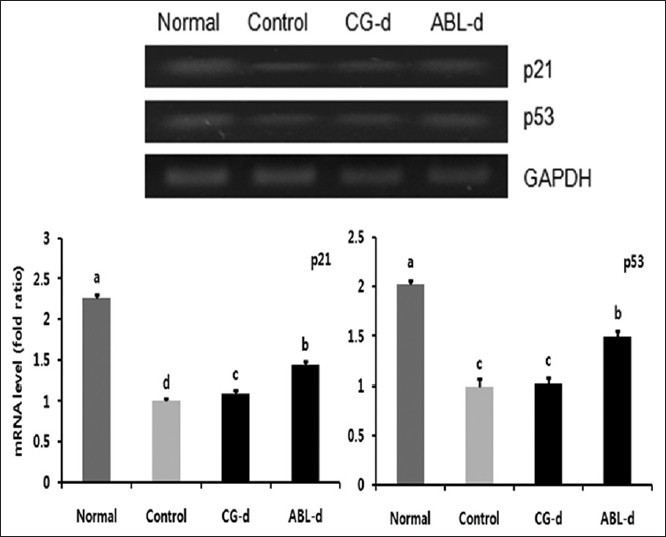 |
Figure 6: Effects of doenjang treatment on messenger RNA expressions of p21 and p53 in the colon tissue of azoxymethane (AOM) and dextran sulfate sodium (DSS) – induced colitis associated cancer mice. Normal, group received phosphate buffered saline (PBS) without AOM and DSS; Control, group received PBS and induced colon cancer by AOM and DSS; CG – d, group received CG – d and induced colon cancer by AOM and DSS; ABL – d, group received ABL – d and induced colon cancer by AOM and DSS. a-cMeans with the different letters are significantly different ( P<0.05) by Duncan’s multiple range test Click here to view |
Discussion
The principal object of this study was to evaluate the potential inhibitory effects of doenjang fermented by probiotic mixed starter cultures in AOM and DSS model of colon carcinogenesis. As mentioned above, we isolated and selected three probiotic microorganisms as starter for preparing meju and doenjang. Then, we manufactured meju and doenjang using the mixed starters and testified the improved quality of the meju and doenjng. In addition, it has been already confirmed the anticancer effect of the meju, which is main ingredient of this doenjang. [15],[16] Therefore, we have attempted to examine the anticancer effect of the end-product of the meju. The results presented herein indicated that doenjang fermented by probiotic mixed starter cultures effectively suppresses AOM and DSS-induced mice colitis-associated colon cancer.
The increase in colon weight to colon length ratio is a typical symptom in mice with colon cancer induced by AOM and DSS and a result of the shortening of colon length and an increase of colon weight. And these phenomena were caused by the stiffness of colonic tissue, the apparent mucosal thickening, and the formation of neoplasia. [20] The increase in colon weight to colon length ratio was intensify in the control group and relieved in doenjang treated group, especially ABL-d treated group. This may means that the dysplastic colonic tissues by the inflammation and tumor and the formation of tumor were alleviated by the administration of doenjang.
As reported previously in many experiments using AOM and DSS-induced colon cancer mice model, the incidence and multiplicity of colonic tumors in mice administered AOM and DSS in this study were quite high. We did observe that doenjang inhibited multiplicity of colonic neoplasms, relieved inflammation and crypt loss in the mucosa. We also detected the apoptosis of colon tumor cells in the doenjang (ABL-d) treated mice by histological study. Many previous studies have shown an anticancer activity of doenjang. [1],[2],[4] These findings suggested that the anticancer effect of doenjang is deeply related with its antiinflammatory and apoptosis-inducing mechanisms.
The mucosal inflammation in colitis and colitis associated colon cancer is caused by hyperactivation of immune cells, which produce high levels of proinflammatory cytokines like IL-6, IL-12, TNF-α, and IFN-γ resulting in colonic tissue damage. [21] These cytokines are important mediators of inflammation and its related cancer and elevated production of proinflammatory cytokines is found in the inflamed colons. [22],[23] Thus, reduction of proinflammatory cytokine production appears to be an effective approach to the prevention and treatment of colitis and colitis associated cancer (CAC). In this study, doenjang especially ABL-d administered mice exhibited significantly lower proinflammatory cytokine levels compared with those from the control group [Figure 3]. This finding suggests that doenjang treated mice were protected from inflammation and its associated cancer.
Inducible nitric oxide synthase and COX-2 are increased in inflamed mucosa and remain elevated in colonic neoplasms. [24],[25] Their over-expression is associated with colon cancer formation and promotion by many potential mechanisms. [26] COX-2 can catalyze the conversion of procarcinogens to carcinogens, indirectly produce free radicals, and promote angiogenesis. On the other hands, iNOS produces excessive NO and it causes DNA damage and posttranslational modification, potentially leading to tumor initiation and promotion. [27],[28] The results of the current study support the concept that inhibitors of iNOS and COX-2 are effective chemopreventive agents against colon carcinogenesis, and doenjang can inhibit colon carcinogenesis in AOM and DSS induced CAC mice model effectively by controlling the expression of iNOS and COX-2.
The Bcl-2 family is the best characterized group of apoptosis mediating factors, which include Bcl-2, Bcl-x, Bax, Bak, and several others. Their biologic functions differentiate into pro-apoptotic (Bax, Bak) or antiapoptosis (Bcl-2, Bcl-xL) properties. The Bcl-2 family related genes regulate cell death and are considered to correlate with the pathogenesis and progression of cancers. [29],[30] p53, a tumor suppressor gene, is known to be a transcriptional regulator of the Bax, and a portion of its tumor suppressor properties may be mediated by transcriptional activation of the Bax. [31],[32] Moreover, the inactivation and/or mutation of p53 are considered a prerequisite for tumor formation and cause a downregulation of p21 gene, a Cdk inhibitor. [33],[34] In this study, pro-apoptotic Bax decreased, and antiapoptotic Bcl-2 significantly increased in the control group and they showed opposite tendencies in doenjang treated group [Figure 5]. The expression of p21 and p53 was reduced in the control group and increased in doenjang, especially ABL-d treatment group [Figure 6]. These results suggest that doenjang (ABL-d) attenuate colitis associated colon cancer inducing cancer cell apoptosis via Bcl-2 family-dependent pathway. And ABL-d may also regulate the expression and mutation of p53 and its downstream death effector Bax and p53-induced cell cycle inhibitor p21.
Conclusion
Doenjang prepared with probiotic mixed starter cultures was shown to attenuate AOM and DSS-induced colitis associated colon cancer in mice. These effects were correlated with an amelioration of colon cancer symptoms such as a reduction of colon weight, reduced numbers of neoplasia. Our data also indicated that the inhibitory effects of doenjang on colon cancer are explained by control of inflammation and cancer cell apoptosis and it is verified by reduced serum proinflammatory cytokine levels of IL-6, IL-12, TNF-α, and IFN-γ, down-regulation of iNOS and COX-2, and up-regulations of p21 and p53 in the colonic tissue. In addition, the effect of doenjang prepared with probiotic mixed starter cultures were stronger than that of commercial doenjang in this study and this tendency was also observed our other in vitro and in vivo test. [15] Therefore, using probiotic mixed starter cultures appears to be an effective method to improve the functionality of doenjang.
Acknowledgment
This research was supported by the grants from the National Institute of Agricultural Science and Technology, Rural Development Administration, Korea.
References
| 1. | Jung KO, Park SY, Park KY. Longer aging time increases the anticancer and antimetastatic properties of doenjang. Nutrition 2006;22:539-45.  |
| 2. | Park KY, Jung KO, Rhee SH, Choi YH. Antimutagenic effects of doenjang (Korean fermented soypaste) and its active compounds. Mutat Res 2003;523-524:43-53.  |
| 3. | Park SK, Seo KI, Choi SH. Quality assessment of commercial doenjang prepared by traditional method. J Korean Soc Food Sci Nutr 2000;9:211-7.  |
| 4. | Choi YH, Choi BT, Lee WH, Rhee SH, Park KY. Doenjang hexane fraction-induced G1 arrest is associated with the inhibition of pRB phosphorylation and induction of Cdk inhibitor p21 in human breast carcinoma MCF-7 cells. Oncol Rep 2001;8:1091-6.  |
| 5. | Kim DH, Lee KH, Yook HS, Kim JH, Shin MG, Byun MW. Quality characteristics of gamma irradiated grain shape improved meju. Korean J Food Sci Technol 2000;32:640-5.  |
| 6. | Ko BK, Kim KM, Hong YS, Lee CH. Metabolomic assessment of fermentative capability of soybean starter treated with high pressure. J Agric Food Chem 2010;58:8738-47.  |
| 7. | Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am J Physiol Gastrointest Liver Physiol 2004;287:G7-17.  |
| 8. | Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn′s disease and ulcerative colitis: Implications for carcinogenesis and prevention. Gut 1994;35:950-4.  |
| 9. | Greenwald P, Milner JA, Anderson DE, McDonald SS. Micronutrients in cancer chemoprevention. Cancer Metastasis Rev 2002;21:217-30.  |
| 10. | Gescher AJ, Sharma RA, Steward WP. Cancer chemoprevention by dietary constituents: A tale of failure and promise. Lancet Oncol 2001;2:371-9.  |
| 11. | Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354-64.  |
| 12. | Müller-Decker K, Fürstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog 2007;46:705-10.  |
| 13. | Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem 2006;387:365-72.  |
| 14. | Danuta MG, Natalia T. Molecular mechanisms of carcinogenesis. In: Wanda BD, editor. Carcinogenesis and Anticarcinogenic Food Components. FL, USA: CRC press; 2006. p. 13-36.  |
| 15. | Jeong JK. Improvement of quality and probiotic effect of meju and doenjang prepared with mixed starter cultures. Doctoral Thesis. Busan, Korea: Pusan National University; 2012.  |
| 16. | Jeong JK, Chang HK, Park KY. Inhibitory effects of meju prepared with mixed starter cultures on azoxymethane and dextran sulfate sodium-induced colon carcinogenesis in mice. J Carcinog 2012;11:30-38.  |
| 17. | Kanneganti M, Mino-Kenudson M, Mizoguchi E. Animal models of colitis-associated carcinogenesis. J Biomed Biotechnol 2011; doi: 10. 11 55 /2011 / 342637.  |
| 18. | Melgar S, Karlsson L, Rehnström E, Karlsson A, Utkovic H, Jansson L, et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol 2008;8:836-44.  |
| 19. | Nita ME, Nagawa H, Tominaga O, Tsuno N, Fujii S, Sasaki S, et al. 5-Fluorouracil induces apoptosis in human colon cancer cell lines with modulation of Bcl-2 family proteins. Br J Cancer 1998;78:986-92.  |
| 20. | Li H, Wu WK, Li ZJ, Chan KM, Wong CC, Ye CG, et al. 2,3′,4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits colitis-associated colorectal carcinogenesis in mice. Br J Pharmacol 2010;160:1352-61.  |
| 21. | Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 2002;15:79-94.  |
| 22. | Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr HA, Hartmann G, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol 2001;281:R1264-73.  |
| 23. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 1998;114:385-91.  |
| 24. | Narayanan BA, Narayanan NK, Simi B, Reddy BS. Modulation of inducible nitric oxide synthase and related proinflammatory genes by the omega-3 fatty acid docosahexaenoic acid in human colon cancer cells. Cancer Res 2003;63:972-9.  |
| 25. | Elder DJ, Baker JA, Banu NA, Moorghen M, Paraskeva C. Human colorectal adenomas demonstrate a size-dependent increase in epithelial cyclooxygenase-2 expression. J Pathol 2002;198:428-34.  |
| 26. | Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009;30:377-86.  |
| 27. | Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol 2001;2:149-56.  |
| 28. | Felley-Bosco E. Role of nitric oxide in genotoxicity: Implication for carcinogenesis. Cancer Metastasis Rev 1998;17:25-37.  |
| 29. | Guo XZ, Shao XD, Liu MP, Xu JH, Ren LN, Zhao JJ, et al. Effect of bax, bcl-2 and bcl-xL on regulating apoptosis in tissues of normal liver and hepatocellular carcinoma. World J Gastroenterol 2002;8:1059-62.  |
| 30. | Que FG, Gores GJ. Cell death by apoptosis: Basic concepts and disease relevance for the gastroenterologist. Gastroenterology 1996;110:1238-43.  |
| 31. | Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995;80:293-9.  |
| 32. | Sturm I, Köhne CH, Wolff G, Petrowsky H, Hillebrand T, Hauptmann S, et al. Analysis of the p53/BAX pathway in colorectal cancer: Low BAX is a negative prognostic factor in patients with resected liver metastases. J Clin Oncol 1999;17:1364-74.  |
| 33. | Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol 2008;14:378-89.  |
| 34. | Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene 2003;22:9030-40.  |
