Anagha A Motgi1, Mrinal V Shete2, Mahesh S Chavan3, Nikkhiel N Diwaan4, Rashmi Sapkal4, Pallavi Channe3
1Department of Oral Medicine and Radiology, D Y Patil Dental School, Dr. D Y Patil Vidyapeeth, Pune, Maharashtra, India
2Department of Oral and Maxillofacial Pathology, D Y Patil Dental School, Dr. D Y Patil Vidyapeeth, Pune, Maharashtra, India
3Department of Oral Medicine and Radiology, D Y Patil Dental College and Hospital, Dr. D Y Patil Vidyapeeth, Pune, Maharashtra, India
4Department of Oral Medicine and Radiology, M A Rangoonwala Dental College, Pune, Maharashtra, India
| Date of Submission | 16-Mar-2021 |
| Date of Decision | 08-Jun-2021 |
| Date of Acceptance | 23-Jun-2021 |
| Date of Web Publication | 07-Oct-2021 |
Correspondence Address:
Anagha A Motgi
Department of Oral Medicine and Radiology, D. Y. Patil Dental School, D Y Patil Knowledge City, Charholi Budruk, Lohegaon, Pune – 412 105, Maharashtra
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.jcar_8_21
Abstract
BACKGROUND: Oral submucous fibrosis (OSMF) is a premalignant condition with a greater prevalence in countries such as India. Various classifications have been put forth by multiple authors to determine the clinical, functional, and histopathological grade of the disease. The classification systems have greatly helped to determine the treatment modality for the patients. Understanding the correlation between the various classifications will help us determine the course of the disease, management, and prognosis of OSMF. This study assesses the correlation between clinical, functional, and histopathological grading of OSMF.
AIM: To assess the correlation between clinical staging, functional staging, and histopathological grading of OSMF.
MATERIALS AND METHODS: Thirty patients with clinical and histopathological diagnosis of OSMF were assigned into clinical stage, functional stage, and histopathological grade. The correlation between these three stages assigned was studied.
STATISTICAL ANALYSIS: The degree of agreement between the clinical, functional, and histopathological classifications was quantified by the Weighted Kappa statistics. Correlation between the three classifications was done using Kendall’s tau and Spearman’s correlation coefficient.
There was a good agreement and statistically significant correlation between clinical and functional grading. There was a poor agreement and no significant correlation between clinical and histopathological grading. There was a poor agreement and no significant correlation between functional and histopathological grading.
Keywords: Clinical staging, functional staging, histopathological grading, oral submucous fibrosis.
| How to cite this article: Motgi AA, Shete MV, Chavan MS, Diwaan NN, Sapkal R, Channe P. Assessment of correlation between clinical staging, functional staging, and histopathological grading of oral submucous fibrosis. J Carcinog 2021;20:16 |
| How to cite this URL: Motgi AA, Shete MV, Chavan MS, Diwaan NN, Sapkal R, Channe P. Assessment of correlation between clinical staging, functional staging, and histopathological grading of oral submucous fibrosis. J Carcinog [serial online] 2021 [cited 2021 Oct 13];20:16. Available from: https://carcinogenesis.com/text.asp?2021/20/1/16/327603 |
Introduction
Oral submucous fibrosis (OSMF) is considered a potentially malignant condition affecting the oral cavity.[1] It was first described in the modern literature by Schwartz in 1952.[2] He coined the term “atrophicaidiopathica mucosae oris” to describe the condition. OSMF is an insidious, chronic disease, characterized by the inflammation and progressive fibrosis of the submucosal tissues.[3] The term “OSMF” was later coined by Joshi in 1953.[4] Other terminologies used for OSMF are “Diffuse OSMF,”[5] “Idiopathic scleroderma of the mouth,”[6] “Idiopathic palatal fibrosis,”[7] and “Sclerosing stomatitis.”[8]
Various studies have proven that areca nut is the primary causative agent in OSMF.[9],[10],[11],[12],[13],[14],[15],[16] The disease is multifactorial in its origin where other possible suggested factors are the deficiency of iron, zinc, and essential vitamins, capsaicin in chillies, etc.[17],[18],[19],[20] Multiple classification systems are proposed by the multiple authors which classified the patients as per their clinical signs and symptoms, functional parameters, and histopathological findings.[21] The staging and grading of OSMF helps to understand the prognosis of the disease and formulate the treatment plan.[22],[23] The current study was performed to assess the agreement and correlation between clinical staging, functional staging, and histopathological grading of patients with OSMF.
Materials and Methods
Thirty patients who reported with clinical signs and symptoms of OSMF at the outpatient department were included in the study after obtaining their informed written consent.
Inclusion criteria
The following diagnostic criteria were selected for enrolling the patients in the study: burning sensation of the oral cavity, dryness of the oral cavity, vesicles or ulceration, reduced mouth opening (normal mouth opening in Indian males – 35–45 mm and females – 30–42 mm), irritation with spicy foods, change in mucosal color, fibrosis, and palpable bands, difficulty in tongue protrusion, difficulty in eating and speaking, and compromised oral hygiene. The patients were selected if they presented with minimum three of the mentioned symptoms.[21]
Exclusion criteria
The patients not willing to participate in the study and the ones already under the treatment for OSMF were excluded from the study. A complete personal history was recorded in a predetermined pro forma, and a written consent was obtained from the patients which included a complete explanation of their present condition, the severity of the condition, the urgent need to stop the habit, and the treatment required. Patients were asked in detail about the form of adverse habit, frequency, and duration of the same.
- Samples for biopsy required for histological grading were taken from all patients. The site of biopsy was posterior buccal mucosa. The formalin-fixed, paraffin-embedded lesions of the oral cavity were referred for histopathological grading. All cases were clinically, functionally, and histologically classified and staged according to the grading systems. The classification given by Gupta D. S in 1980 was referred for classifying patients as per the clinical stages. The patients were classified into four stages: very early stage, early stage, moderately advanced stage, and advanced stage.[24] Following are the criteria for clinical grading according to severity:
Grade 1 – Incipient/very early stage
Burning sensation, dryness of the mouth, vesicles or ulceration, irritation with spicy foods, no change in mucosal color, no fibrosis, no bands palpable, normal mouth opening (44 mm), normal tongue protrusion, eating and speaking not affected, and oral hygiene not affected.
Grade 2-Mild
Burning sensation, dryness of the mouth, irritation with spicy foods, mucosa blanched and loss of elasticity, no clear-cut fibrotic bands, slight restriction in mouth opening (26–30 mm), normal tongue protrusion, eating and speaking not affected, oral hygiene not affected.
Grade 3-Moderate
Burning sensation, dryness of the mouth, irritation with spicy foods, blanched, opaque, leather like mucosa, vertical fibrotic band on buccal mucosa making it stiff, considerable reduction in mouth opening (15–25 mm), tongue protrusion not much affected, difficulty in eating and speaking, poor oral hygiene.
Grade 4 a-advanced stage
Burning sensation and dryness of the mouth, irritation with spicy foods, blanched, opaque leather like mucosa, thick fibrosed bands on the buccal mucosa in retromolar area and at pterygo-mandibular raphe, marked reduction in mouth opening (2–15 mm), restricted tongue protrusion, eating and speech very much impaired, and very poor oral hygiene.
Grade 4b-Advanced premalignant and malignant change
It shows classical signs of OSMF and is associated with leukoplakia and lichen planus.
The classification given by Bailoor D.N. in 1993 was referred for classifying patients as per the functional stages.[25] The patients were classified into Stage I (early OSMF), Stage II (moderate OSMF), and Stage III (severe OSMF). Measurement of mouth opening, tongue protrusion, and cheek flexibility (CF) were used as the criteria for functional classification.
Stage 1-Early oral submucous fibrosis
(1) No restriction in mouth opening, normal distance between central incisor tips – males– 35–45 mm, females 30–42 mm. (2) No restriction in tongue protrusion, normal mesioincisal angle of upper central incisor to tip of the tongue when maximally extended with mouth wide open – males – 5–6 mm, females– 4.5–5.5 mm. (3) CF = V1-V2, V1 = V1 is marked at 1/3rd distance from angle of mouth in the line joining the tragus and angle of mouth, V2 = subject is then asked to blow his cheeks fully and distance is measured between two points marked on the cheek. Mean value – males – 1.2 cm, females 1.08 cm
Stage 2-Moderate oral submucous fibrosis
(1) Mouth opening is reduced by approximately 33% (2) Reduction in tongue protrusion (3) Reduction in CF.
Stage 3-Severe oral submucous fibrosis
(1) More than 66% reduction in mouth opening (2) More than 66% reduction in tongue protrusion, tongue may appear fixed (3) More than 66% reduction in CF.
Method for measurements[25]– (1) mouth opening [Figure 1]a and [Figure 1]b. This was assessed as the interincisal distance as measured from the mesio-incisal edge of the upper left central incisor tooth to the mesio-incisal edge of the lower left central incisor tooth. The measurement was made using a geometric divider and scale and was recorded in millimeters. (2) Tongue protrusion [Figure 2]a and [Figure 2]b: The degree of protrusion was recorded in millimeters from the incisal edge of the lower teeth. This was done by viewing the protruded tongue from the lateral aspect of the head and measuring the distance from the mesial contact area of the lower central incisors to the tip of the protruded tongue. (3) Burning sensation: Burning sensation was recorded as per a Verbal Analog Scale where values were recorded on a scale of 0–10. Patients were explained the scale in the native language and asked to mark the appropriate unit on the scale as per the intensity of burning. This was measured at baseline and as per the regular follow-up protocol in both the groups. (4) CF [Figure 3]a, [Figure 3]b, [Figure 3]c, [Figure 3]d = V1-V2. V1 = V1 is marked at 1/3rd distance from angle of mouth and the line joining the tragus and angle of mouth, V2 = subject is then asked to blow his cheeks fully and distance is measured between two points marked on the cheek. Mean value in males is 1.2 cm and 1.08 cm in females.
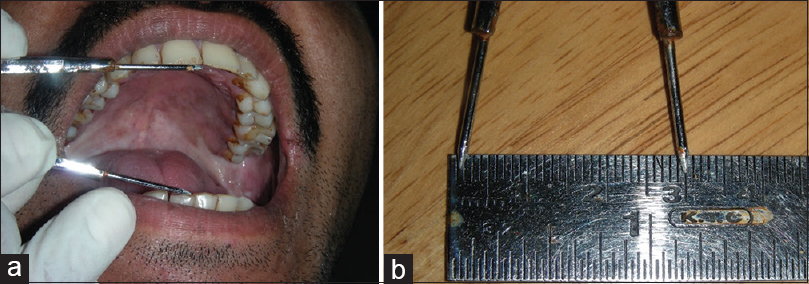 |
Figure 1: (a) Measurement of mouth opening. (b) Measurement of mouth opening Click here to view |
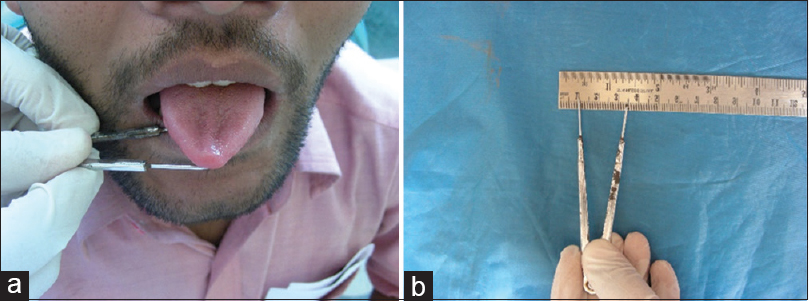 |
Figure 2: (a) Measurement of tongue protrusion. (b) Measurement of tongue protrusion Click here to view |
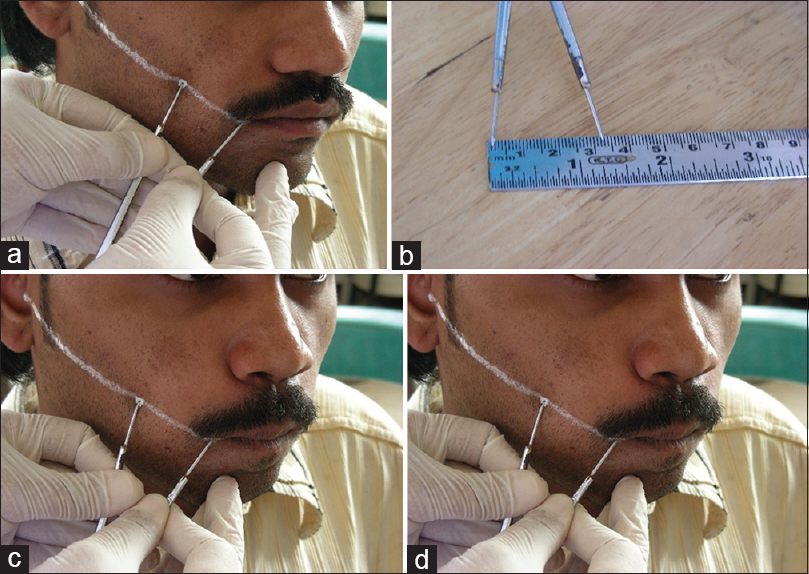 |
Figure 3: (a) Measurement of V1. (b) Measurement of V1. (c) Measurement of V2. (d) Measurement of V2 Click here to view |
The classification based on the clinical and histopathological findings given by Khanna J.N. and Andrade N.N. was referred for classifying patients as per the histopathological grades.[26] The patients were classified into four groups: Group I (very early cases), Group II (early cases), Group III (moderately advanced cases), Group IV A (advanced cases), and Group IV B (advanced cases).
Group 1-Very early cases
- Fine, fibrillar collagen network interspersed with marked edema
- Blood vessels dilated and congested
- Large aggregates of plump, young fibroblasts present with abundant inflammatory cells mainly consisting of polymorphonuclear leukocytes with few eosinophils
- Epithelium normal.
Group 2: Early cases
- Juxtaepithelial hyalinization present
- Collagen present as thickened but separate bundles
- Blood vessels dilated and congested
- Inflammatory cells mainly consist of polymorphonuclear leukocytes with few eosinophils and occasional plasma cells
- Flattening or shortening of epithelial rete pegs evident with varying degree of keratinization.
Group 3: Moderately advanced cases
- Juxtaepithelial hyalinization present
- Thickened collagen bundles faintly discernible, separated by very slight, residual edema
- Blood vessels mostly constricted
- Mature fibroblasts with scanty cytoplasm and spindle-shaped nuclei
- Inflammatory exudates consist mainly of lymphocytes and plasma cells
- Epithelium markedly atrophic with loss of rete pegs
- Muscle fibers seen interspersed with thickened and dense collagen fibers.
Group 4-A and B
Advanced cases and advanced cases with premalignant and malignant changes
- Collagen hyalinized as smooth sheet
- Extensive fibrosis-obliteration of mucosal blood vessels and elimination of melanocytes
- Fibroblasts markedly absent within hyalinized zones
- Total loss of epithelial rete pegs
- Mild to moderate atypia present
- Extensive degeneration of muscle fibers evident
- (√ = key points in clinical and histopathological grading)
Statistical analysis
The data were collected and evaluated. It was used to correlate between all the classification systems and to determine its diagnostic and prognostic value. The data were entered in MS excel and analyzed with statistical software Epi info and Primer of Biostatistics. In the present study, the degree of agreement between the clinical, functional, and histopathological classifications was quantified by the Weighted Kappa (K) statistics. Cohen’s K was calculated using Medcalc Version 11.1.0 (MedCalc Software bvba, Broekstraat 52, 9030 Mariakerke, Belgium). Correlation between the three classifications was done using Kendall’s tau and Spearman’s correlation coefficient. SPSS software version 10.0 was used for calculating the correlation coefficients. Probability value of < 0.05 was considered statistically significant.
Results and Observations
The present study was undertaken to assess the correlation between clinical staging, functional staging, and histopathological grading of patients with OSMF. A total of 30 patients were included in the study. The study sample included the patients with age groups of 20–70. Out of 30 patients, 20 patients were in the category of the age 20–30 (67%). Seven patients were in the age limit of 30–40 (23%) and 3 in 60–70 group (10%). All the 30 patients were grouped according to their tissue abuse habits. Gutkha was consumed by five patients (17%), gutkha in combination with tobacco was consumed by 16 patients (54%), betel nut and smoked form of tobacco, i.e. cigarette by two patients (7%). Only tobacco in quid form was consumed by one patient (3%) and four patients presented with a combination of the mentioned habits.
A total of 30 patients were graded according to functional and clinical criteria, among whom a total of 22 patients, the functional grading was similar to the clinical staging [Table 1]. Hence, the percentage of agreement was 73%. No agreement or poor agreement was seen in 8 cases. Overall, there was a good agreement between clinical and functional grading. There was a statistically significant correlation between clinical and functional grading [Table 2].
| Table 1: Clinical and functional staging assigned to the patients Click here to view |
| Table 2: Agreement and correlation between clinical and functional classification Click here to view |
Out of 30 patients, only eight patients presented clinical staging similar to histopathological grading [Table 3]. Twenty-two patients presented with no agreement or poor agreement. Hence, the percentage of agreement was 26.6%. There was a poor agreement between clinical and histopathological grading. There was no significant correlation between clinical and histopathological grading [Table 4].
| Table 3: Clinical and histopathological staging assigned to the patient Click here to view |
| Table 4: Agreement and correlation between clinical and histopathological classification Click here to view |
Out of 30 patients, functional grading was similar to histopathological staging in only 12 patients [Table 5]. No agreement or poor agreement was seen in 18 patients. The percentage of agreement was only 40%. There was a poor agreement between functional and histopathological grading. There was no significant correlation between functional and histopathological grading [Table 6].
| Table 5: Functional and histopathological staging assigned to the patients Click here to view |
| Table 6: Assessment and correlation between functional and histopathological classification Click here to view |
Since there were only two females (small sample size to make any meaningful comparison) statistical test has not been carried out. Similarly, the type of habit comparison with each grading and staging is not done due to a smaller number of cases in each sub-category being compared.
Discussion
The present study was undertaken to assess the correlation between clinical staging, functional grading, and histopathological grading of the patients with OSMF. A total of 30 patients were included in the study, of which 28 patients were male and 2 were female. A higher male predilection for OSMF has been reported in the literature, and the findings in this study conform to the studies published by Lai et al. and Kumar et al. where a male predilection of as high as 96.67% and 100%, respectively, has been reported.[27]
Most of the patients were between 20 and 30 years of age group, a finding that concurred with that of Maher et al. who reported that 70% of males were <30 years of age.[14] In a study conducted in 2006 by Kumar et al., 70.69% of male patients were below 30 years of age.[27]
All patients in the present study gave a positive history of areca nut chewing in the raw form as betel nut proper or as in the form of a commercial preparation called gutkha or pan masala which is proven to be a major causative agent of OSMF.[28] Much has been published in the literature about the development of OSMF due to betel nut chewing habit and the first line of treatment being stoppage of its consumption before and after any therapy.
The most common form of areca nut used was found to be gutkha, with 76.6% (23 out of 30) patients using it this way, this finding corresponds with the study by Kumar et al.[27] A higher prevalence of betel nut chewing was observed in the present study in females as opposed to males. However, a higher prevalence of gutkha was observed in males.
All the types of the habits were noted. Five patients gave a history of tobacco chewing habit. Sixteen patients used gutkha and tobacco in combination. Two patients chewed only betel nut and two patients only smoked form of tobacco. One patient consumed only tobacco and four patients used combination of all the mentioned habits.
Clinical staging
All the 30 patients were subjected for clinical staging according to the classification criteria. Eight patients out of 30 were staged as Stage 1. Twelve patients were assigned a Stage 2. The Stage 3 was assigned to nine patients. The remaining category was the Stage 4 which had only one patient.
Functional staging
The functional staging was done for all the patients. Four patients presented with Stage 1 features. Seventeen patients were grouped under the Stage 2. The Stage 3 was assigned to nine patients.
Histopathological grading
Histopathological examination was done for all the patients after incisional biopsies were taken. One patient was included in Grade 1, 11 under Grade 2, 12 under Grade 3 and 6 were included under the Grade 4.
Modak et al.[29] conducted a comparative study of clinical staging of OSMF in 2015. As per their observations, the correlation between clinical and functional staging was not significant (P > 0.05), whereas the comparison of the functional staging with histopathological staging was more reliable (P < 0.01) as an indicator to assess the severity of the disease rather than clinical staging. In our study, there was a good agreement and statistically significant correlation only between clinical and functional grading. Another study was carried out by Bhatt et al.[30] in 2018. They found definitive correlation between functional and histological stages of OSMF. They suggested that clinically advanced stages have extensive fibrosis histologically. Radhika et al.[31] conducted a study on OSMF patients and concluded that there was significant correlation between functional and histopathological stages of OSMF. The comparison between the functional and histological stages was more reliable in the determination of severity of the disease than the clinical staging. In our case, significant correlation was observed between clinical and functional stages. Similar to the study mentioned above, another study conducted by Shivakumar and Sahana[32] also highlights about significant correlation between functional and histological staging of OSMF. Biradar et al.[33] suggested that the clinical and histological gradings of OSMF have great correlation and clinically advanced cases show severe and extensive fibrosis. Similar to our findings, Pandya et al.[34] also found that there was no significant correlation between the clinical and histological grades of OSMF. They only found that histological grade worsened with the severity and duration of the tissue abuse habit. The statistical analysis of the study performed by Goel et al.[35] also showed no significant correlation between the clinical and histological staging of OSMF.
As per our observation, the signs and symptoms manifested clinically have a direct impact on the normal functional abilities such as mouth opening, tongue protrusion, and CF. Hence, the patients with blanching of oral mucosa, burning sensation of oral cavity, etc., need to be evaluated for discrepancies in the above-mentioned functional. They can be demonstrated to the patient to highlight the functional loss and this may help the patient to motivate for seeking treatment.
Conclusion
The present study was undertaken to assess the correlation between clinical staging, functional staging, and histopathological grading of OSMF. There was a good agreement and statistically significant correlation between clinical and functional grading. There was a poor agreement and no significant correlation between clinical and histopathological grading. There was a poor agreement and no significant correlation between functional and histopathological grading. Age did not show any significant correlation with the three classifications.
As there is some correlation between the various classification systems, each patient with OSMF should be thoroughly evaluated clinically, functionally, and histopathologically. This will aid in accurate diagnosis, classification, estimation of prognosis, and treatment of the patient. As clinical and functional grading show a good degree of correlation, we hope that this in the future will enable us to draw an inference on the prognosis of the disorder.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. | |
| 25. | |
| 26. | |
| 27. | |
| 28. | |
| 29. | |
| 30. |
Bhatt P, Manjunath M, Khakhla D, Gubrellay P, Bhargava R, Guruprasad L. Assessment and correlation between functional and histological staging of oral submucous fibrosis: A clinicohistopathologic study. Natl J Maxillofac Surg 2019;10:27-32.
 [PUBMED] [Full text] |
| 31. | |
| 32. |
Shivakumar G, Sahana S. Correlation between the functional and histological staging of oral submucous fibrosis. J Indian Acad Oral Med Radiol 2010;22:133-5.
 [Full text] |
| 33. | |
| 34. | |
| 35. |
Figures
[Figure 1], [Figure 2], [Figure 3]
Tables
[Table 1], [Table 2], [Table 3], [Table 4], [Table 5], [Table 6]
