Ashok Kumar Das1, Nizara Baishya1, Anupam Sarma2, Amal Chandra Kataki3, Avdesh Kumar Rai4, Chandi Ram Kalita5
1 Department of Head and Neck Oncology, Dr. B. Borooah Cancer Institute, Guwahati, Assam, India
2 Department of Pathology, Dr. B. Borooah Cancer Institute, Guwahati, Assam, India
3 Department of Gynae Oncology, Dr. B. Borooah Cancer Institute, Guwahati, Assam, India
4 Department of Biotechnology, Dr. B. Borooah Cancer Institute, Guwahati, Assam, India
5 Department of Biostatistics, Dr. B. Borooah Cancer Institute, Guwahati, Assam, India
| Date of Submission | 11-Dec-2018 |
| Date of Acceptance | 01-Apr-2019 |
| Date of Web Publication | 09-May-2019 |
Correspondence Address:
Nizara Baishya
Department of Head and Neck Surgery, Dr. B. Borooah Cancer Institute, Guwahati, Assam
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_24_18
Abstract
INTRODUCTION: Nasopharyngeal cancer is not a common disease in most parts of the world. In India also, nasopharyngeal carcinoma (NPC) is not a common cancer, except for the Northeastern region of the country. Expression of matrix metalloproteinase 9 (MMP9) in the tumor cells is related to tumor invasion and metastasis. The aim of the present study is to analyze the expression of MMP9 in NPC and evaluate its prognostic implications.
MATERIALS AND METHODS: A total of 32 histologically confirmed tissue samples of NPC were examined by immunohistochemical staining to assess the expression of MMP9. Clinicopathological parameters and levels of MMP9 expression in the tumor tissue were analyzed using Chi-square test. Survival analysis was done using the Kaplan–Meier method and was compared using log-rank test. P <0.05 was considered statistically significant.
RESULTS: Of the 32 tissue samples of NPC, 23 (71.9%) were male and 9 (28.1%) were female. 7 (21.9%) patients presented in T1 Stage, 8 (25.0%) in T2, 12 (37.5%) in T3, and 5 (15.6%) in T4 Stages, respectively. 29 (90.6%) patients presented with lymph node metastasis. MMP9 expression level was significantly correlated with patient’s age (P = 0.033), tumor histology (P = 0.017), tumor stage (P = 0.021), and lymph node metastasis (P = 0.011). The 5-year overall survival is higher for low-level expression as compared to high-level expression of MMP9 (P = 0.046).
CONCLUSION: MMP9 is an important prognostic factor for NPC. High expression of MMP9 is associated with cervical lymph nodes metastasis and poor survival outcome.
Keywords: Immunohistochemistry, matrix metalloproteinase 9, nasopharyngeal carcinoma, Northeast, prognosis
How to cite this article:
Das AK, Baishya N, Sarma A, Kataki AC, Rai AK, Kalita CR. Assessment and clinicopathological correlation of matrix metalloproteinase 9 expression in nasopharyngeal carcinoma. J Carcinog 2019;18:1-6
How to cite this URL:
Das AK, Baishya N, Sarma A, Kataki AC, Rai AK, Kalita CR. Assessment and clinicopathological correlation of matrix metalloproteinase 9 expression in nasopharyngeal carcinoma. J Carcinog [serial online] 2019 [cited 2021 Oct 13];18:1-6. Available from: https://carcinogenesis.com/text.asp?2019/18/1/1/257900
Introduction
Nasopharyngeal carcinoma (NPC) is a rare malignancy in most regions of the world. It is a common cancer in South China, Southeast Asia, among the Maghrebian Arabs in North America, and Eskimos in the Arctic with a remarkable racial and geographical distribution.[1] NPC is uncommon in most other regions of India except the Northeastern (NE) hilly states mainly Nagaland, Mizoram, and parts of Arunachal Pradesh. According to the National Cancer Registry Programme of India, both in males and in females, Nagaland Cancer Registry has recorded the highest age-adjusted incidence rates of NPC.[2] The striking feature of NPC is that the incidence is as low as 0.5/100,000–2.0/100,000 among Caucasoid to as high as ∼20/100,000 among the Cantonese/Zhongshan dialect Chinese. The NE region has been immigrated by the Tibeto-Burman population, so Mongoloid features were found among the people of this region. Certainly, most of the NPC patients present in a fairly advanced stage because of its deep anatomical location and initial vague symptoms. Studies had shown that the Naga community belongs to a tribe of Mongolian origin with different cultural and dietary habits. Thus, it can be inferred that NPC is relatively higher among the Mongoloid population in the NE region of India as compared to the Caucasian population residing in the other parts of the country.[3] NPC has a predilection for regional lymph node metastasis. Approximately, 90% of NPC patients presented with cervical lymph node metastasis.[4] It is known that the clinical stage at the time of diagnosis and the anatomical factors of the tumor such as localization of the lymph and blood vessels along with the performance status were considered to be the important predictors related to disease prognosis. Hence, useful markers associated with biological aggressiveness are needed to predict the outcome of the disease. Furthermore, prognostic markers could also serve in developing new treatment strategies in these groups of cancers.
The matrix metalloproteinases (MMPs) are a large group of secreted proteinases that require zinc for catalytic activity. MMP9 plays a critical role in the breakdown of extracellular matrix resulting in tumor invasion, metastasis, and induction of tumor tissue vascularization.[5] In 1962, expression of MMP was first discovered in tumor cells, and since then, more than 20 kinds of MMPs have been found. Association of MMP9 with metastasis in cancers such as the lung, prostate, breast, colon, head, and neck were reported earlier, but there is sparse of published data on association of MMP9 expression in NPC.
The prognostic role of MMP9 is still unclear. However, its prognostic role in the tumor progression of NPC was studied by correlating the MMP9 expression to clinical behavior of the disease.
Materials and Methods
Patients
Newly diagnosed patients of NPC attending the tertiary cancer center of Northeast India for treatment during the period from June 2012 to May 2013 were recruited into the study. The patients were followed up for a minimum of 5 years. Thirty-two paraffin-embedded tissue samples of histologically proven squamous cell carcinoma (SCC) of NPC were available for this study. Patient without any prior treatment history was recruited in this study. The study protocol was approved by the Institutional Ethics Committee and Informed consents were obtained from the patients.
The mean age of the patients was 44 years (minimum 15 and maximum 78 years). The stage of the disease, tumor size, and lymph node involvement were determined according to the 7th Edition of the American Joint Cancer Committee. The histological grade of the tumors was reviewed and classified according to the WHO Classification of NPC.
The treatment strategies for patients in this series were carried out according to the evidence -based standard protocol for treatment, and the treatment line depends on the stage of the tumor. Radical radiotherapy (RT) (50–60 Gy) with concomitant chemotherapy (CT) was the treatment for 20 of the 32 patients, 10 patients were treated by neoadjuvant CT followed by RT + CT. Two of the patients had an advanced carcinoma, and they received only palliative RT (30 Gy). In the follow-up, the patients were followed up every 3–6 months by both clinical and telephonic calls to assess the locoregional tumor control and survival, respectively.
Immunohistochemistry
Immunohistochemistry was performed on 4-μm thick sections made from 10% formalin-fixed paraffin-embedded specimens. One representative section from tumor block was incubated with biotin-labeled rabbit antigoat antibody for 10 min at room temperature, and subsequently were incubated with streptavidin-conjugated horseradish peroxidase. 3, 3′-Diaminobenzidine (DAB) chromogen solution in DAB buffer substrate was used to develop peroxidase reaction. Sections were analyzed using a bright-field microscope by counterstaining with hematoxylin and then mounted with neutral gum.
Evaluation of staining
Tissue sections that were stained immunohistochemically were reviewed and scored by the pathologist being blinded to the clinical parameters. Wang et al. described the staining intensity of tumor cells in colorectal cancer.[6] The extent of the staining is defined as the percentage of positively stained tumor cells or normal nasopharyngeal epithelial cells in relation to the whole tissue area. It was scored on a scale of 0–4 as the following: 0, <10%; 1, 10%–25%; 2, 26%–50%; 3, 50%–75%; and 4, >76%. The final staining score for MMP9 is the summation of the staining-intensity and staining-extent scores which ranges from 0 to 7. A final staining scores ranging from 0 to 5 and 6–7 were considered as low and high expression, respectively, for statistical analysis.
Statistical analysis
The data were analyzed using (SPSS) version 19 software (SPSS 19.0, IBM Inc., Chicago, IL, USA). The correlations of tumor stage, Tumor-Node-Metastasis classification, histological grade, presence of nodal metastasis, and age were analyzed separately according to the expression of MMP9 in tumor cells. The statistical significance of these correlations was determined with the Chi-square test. The overall survival (OS) rates were analyzed using the Kaplan–Meier method, and the statistical differences in survival among the groups were compared by log-rank test. The 5-year OS was defined as the time calculated from the date of first diagnosis to the date of death caused by NPC or the date of the last contact. P < 0.05 is considered as statistically significant.
Results
Patients clinicopathological parameters
A total of 32 patients with SCC of NPC were included in the study. Of 32 patients’ cohort, 23 (71.9%) were male and 9 (28.1%) were female (male:female was 2:1). The median age of presentation was 44 years, with a range from 15 to 78 years. 20 (62.5%) patients were in the age group of 50 years or less and 12 (37.5%) were above 50 years. Of 32 patients, 9 (28.1%) patients were diagnosed histologically as keratinizing SCC, 14 (43.8%) with nonkeratinizing SCC, and the remaining 9 (28.1%) with undifferentiated carcinoma. On clinical and radiological examination, 7 (21.9%) tumors were classified as T1, 8 (25.0%) as T2, 12 (37.5%) as T3, and 5 (15.6%) as T4. 29 (90.6%) patients presented with clinically positive cervical lymph nodes, and 3 (9.4%) patients had no regional lymph node metastasis. The clinical stages at the time of diagnosis were 5 (15.6%) in Stage II, 19 (59.4%) in Stage III, and 8 (25.0%) in Stage IV [Table 1].
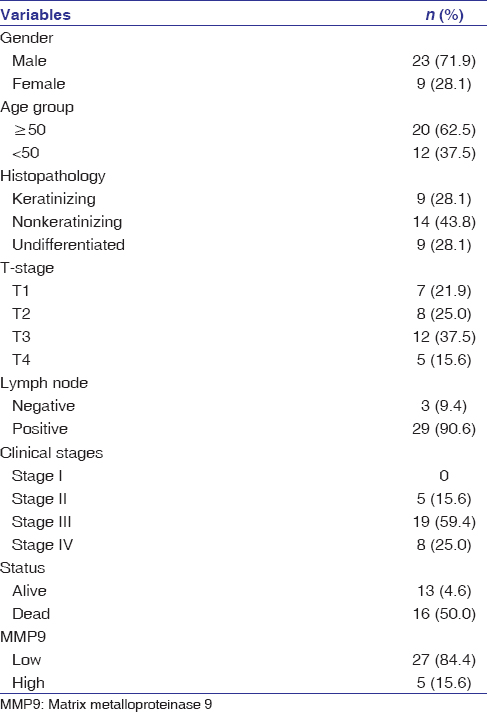 |
Table 1: Clinicopathological parameters of patient’s with nasopharyngeal carcinoma Click here to view |
Relationship between clinicopathological parameters and matrix metalloproteinase 9 expression
The relationship between clinicopathological parameters and MMP9 expression levels of the patients with NPC has been summarized in [Table 2]. We observed that MMP9 expression level was significantly correlated with patient’s age (P = 0.033), tumor histology (P = 0.017), tumor stage (T classification) (P = 0.021), and lymph node metastasis (N classification) (P = 0.011) [Table 2]. However, patient’s gender and clinical stages were not significantly associated with MMP9 expression level (P > 0.05).
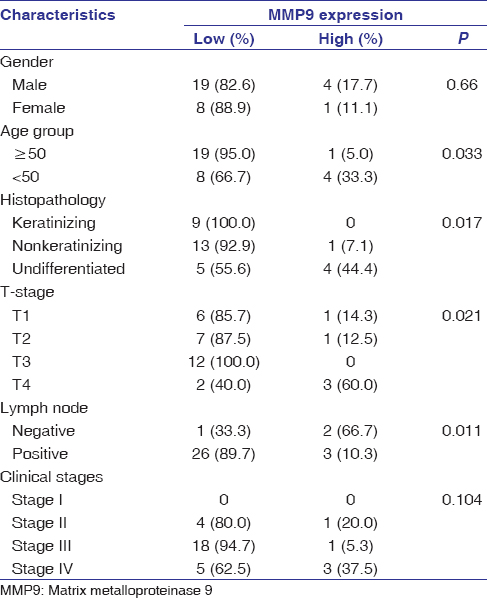 |
Table 2: Correlation of matrix metalloproteinase 9 expression with various clinical characteristics Click here to view |
Matrix metalloproteinase 9 expression and overall survival
Of 32 patients, 16 (50.0%) died and 16 (50.0%) patients were either alive or censored at the end of follow-up. Kaplan–Meier method with log-rank test showed that the 5-year OS was higher in patients with low level of MMP9 expression than those with high level of MMP9 expression (P = 0.046). [Table 3] showed the median survival of patients in relation to the MMP9 expression group. The 5-year OS in patients with low MMP9 expression was 31.5% with a median survival of 49 months (95% confidence interval (CI) 36.7–61.3). However, all the patients with high level of MMP9 expression were dead before completion of the follow-up period. The median survival for the group with high level of MMP9 expression was 25 months (95% CI 14.3–35.7)[Figure 1].
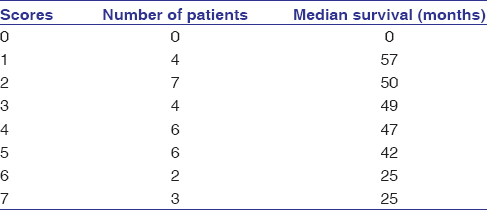 |
Table 3: Median survival of patients in relation to matrix metalloproteinase 9 expression group Click here to view |
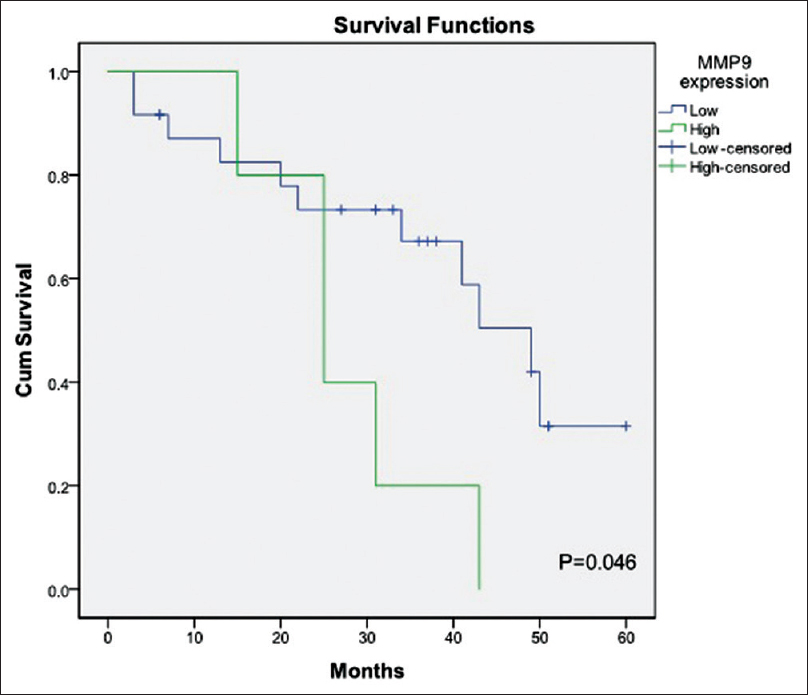 |
Figure 1: Five-year overall survival between high-level and low-level expression of MMP9 Click here to view |
Discussion
NPC is a rare malignancy in most regions of the world, with a remarkable racial and geographical distribution affecting South China, Southeast Asia, the Maghrebian Arabs in North America, and Eskimos in the Arctic. Due to the anatomical location and vague clinical symptoms, it is often misdiagnosed leading to late presentation. The complex interactions of genetic, viral, environmental, and dietary factors might be associated with the etiology of NPC and has attracted worldwide attention in recent decades. In most of the studies reported from different parts of the world, it was seen that males were more affected than females.[7] Studies from the NE region of India had shown different age group peaks with a male preponderance (M: F = 3:1).[3] This similar finding was observed in our cohort as well. NPC shows a bimodal age distribution in different parts of the world. However, no specific bimodal age peaks were observed in a study from the NE region of India.[8] In our series, the age of the patients ranged from 15 to 78 years with a median of 44 years. Twenty (62.5%) patients were in the age group of 50 years or less and 12 (37.5%) were above 50 years which shows a bimodal age distribution supporting the other studies. NPC has been thought to be closely associated with Epstein–Barr virus infection, dietary, and genetic factors. The risk of developing NPC increases by 2–6 times if there is a history of exposure to steam, dust fumes, and chemical gases in the workplace for a long period. Nonkeratinizing SCC was the most common histological variant found in our study. Most of the patients present in Stage III and IV because of the vague symptoms and ignorance about the disease. 90.6% of patients presented with cervical lymph node metastasis. MMP9 is a Zn2+-dependent endopeptidase that mediates the degradation of extracellular matrix protein[9] and is associated with tumor invasion and metastasis.[10],[11] In general, MMP9 expression is produced not only by tumor cells but also a variety of stroma cells and is predominantly induced by tumor cells.[12] It is synthesized and secreted in monomeric form as zymogen and belongs to the gelatinase subgroup. Zhang et al. reported that MMP9 expression was significantly associated with lymph nodes metastasis and histology classification in NPC.[13] This finding was also in our study. In our study, high expression of MMP9 was found in 5 out of 32 NPC samples (15.6%). High expression value of MMP9 in our study was mostly found in tumors with histology of undifferentiated type (Type III), which is in contrast to the study by Farhat et al. who found overexpression of MMP9 in nonkeratinizing variety.[14] Increased expression of MMP9 is usually seen in invasive and metastatic cancers such as colorectal cancer,[15] gastric carcinoma,[16] pancreatic carcinoma,[17] breast cancer,[18] and oral cancer.[19]
We did not find a significant association of MMP9 levels with gender and clinical stage in 32 tissue samples of NPC. However, we observed that the expression level of MMP9 was significantly correlated with age which is in contrast to the study by Liu et al.[9] In our study cohort of 32 samples, it has been observed that high level of MMP9 expression was significantly associated with T classification (tumor size) and N classification (lymph node metastasis) like Liu et al.[9] However, the association of MMP9 levels with the clinical stages was not found in our series which is similar to the study by Cho WC et al.[5] These findings suggest that high level of MMP9 expression play an unfavorable role in the pathogenesis of NPC leading to poor survival. Liu et al. had correlated the level of MMP9 expression with patients’ OS and found that the patients with higher expression had shorter survival time.[9] We have also observed in our study that patients with higher expression of MMP9 had shorter survival time than the patients with low expression.
There are certain limitations in the present study as the sample size is small and the information on disease-free survival could not be obtained as most of the patient’s follow-ups were done by telephonic calls. However, this is the first study from the NE India to investigate the expression of MMP9 as the prognostic markers for NPC.
Conclusion
The high expression of MMP9 in patients with NPC was mostly found in advance Stage (III and IV). This suggests that MMP9 expression is an important prognostic factor for NPC. However, further follow-up study using a comparison/control studies would be needed to verify these findings regarding the role of MMP9 in NPC that can be used to provide optimal therapy.
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving the human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Acknowledgment
This work was supported by Department of Biotechnology (DBT), Government of India-sponsored project and from an intramural grant from the Institute of Life Sciences, Bhubaneswar, India. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support and sponsorship
This study was funded by DBT, Government of India.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. |

