Soley Bayraktar1, Sameer Batoo2, Scott Okuno2, Stefan Glück3
1 Department of Medicine, Division of Medical Oncology and Hematology, Mayo Clinic Health System, Eau Claire, WI, USA; Department of Medicine, Division of Medical Oncology and Hematology, Biruni University School of Medicine, Istanbul, Turkey
2 Department of Medicine, Division of Medical Oncology and Hematology, Mayo Clinic Health System, Eau Claire, WI, USA
3 Vice President Global Medical Affairs, Early Assets, Celgene Corporation, Summit, NJ, USA
| Date of Submission | 02-Mar-2019 |
| Date of Acceptance | 09-Apr-2019 |
| Date of Web Publication | 23-May-2019 |
Correspondence Address:
Soley Bayraktar
Mayo Clinic Health System, Albert J. And Judith A. Dunlap Cancer Center, 1221 Whipple St., Eau Claire, WI 54702
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jcar.JCar_2_19
Abstract
The idea of using the immune system to fight cancer is over 100 years old. A new molecular approach led to a better understanding of the immune system. Checkpoint regulation, understanding the roles of Tregs, Th1, and Th2, development of Chimeric antigen receptor (CAR)-T cells, as well as regulation of dendritic cells and macrophages, are just a few examples of our understating that has also led to the discovery of immune checkpoint inhibitors (ICIs) and modulators. This led the Nobel Prize committee in 2018, to award Dr. James P. Allison the Nobel Prize in medicine for the discovery of Cytotoxic T-lymphocyte-associated antigen-4, and Dr. Tasuku Honjo for the discovery of programmed cell death-1 (PD-1)/PD-1-ligand (PDL-1). Several ICIs are already approved by the regulatory authorities, and many more are currently used in studies of several solid tumors and hematologic malignancies. Positive studies have led to the US Food and Drug Administration (FDA) and European Medicines Agency approval of a number of these compounds, but none to date are approved in breast cancer (BC). Moreover, PD-1/PDL-1, MSI high (and dMMR), and tumor mutational burden are the currently “best” predictive markers for benefit from immunotherapy. BCs have some of these markers positive only in subsets but less frequently expressed than most other solid tumors, for example, malignant melanoma or non-small cell lung cancer. To improve the potential efficacy of ICI in BC, the addition of chemotherapy was one of the strategies. Many early and large clinical trials in all phases are underway in BC. We will discuss the role of immune system in BC editing, and the potential impact of immunotherapy in BC outcomes.
Keywords: Breast cancer, checkpoint inhibitors, cytotoxic T-lymphocyte-associated antigen-4, immunotherapy, programmed cell death-1, programmed cell death ligand-1
How to cite this article:
Bayraktar S, Batoo S, Okuno S, Glück S. Immunotherapy in breast cancer. J Carcinog 2019;18:2
How to cite this URL:
Bayraktar S, Batoo S, Okuno S, Glück S. Immunotherapy in breast cancer. J Carcinog [serial online] 2019 [cited 2021 Oct 13];18:2. Available from:
Introduction
Evading immune destruction is an emerging hallmark of cancer. The immune system plays a dual role in cancer: it not only can suppress tumor growth by destroying cancer cells or inhibiting their outgrowth but also promotes tumor progression either by selecting for cancer cells that are more fit to survive in an immunocompetent host or by establishing conditions within the tumor microenvironment that facilitate tumor outgrowth. Nonetheless, numerous studies have shown that tumors can be recognized and contained for extended periods by the immune response through the concerted action of the innate (via chronic inflammation orchestrated by the innate immune system) and adaptive immune responses.[1] Despite these efforts, often cancer still develops, at increased frequency with age, as a consequence of selecting less immunogenic tumor cells, or the increased effectiveness of tumor-mediated immunosuppression (immune subversion) or both.[2],[3]
Our understanding of the complex interplay between cancer and the immune system has improved substantially by moving from the concept of “immune surveillance”[4] to that of “immunoediting.”[3] The concept of cancer immunosurveillance was first proposed in 1909 by Ehrlich who suggested that evolving tumors are constantly identified and eradicated by the host immune system even before clinical manifestations occur. This concept was refined by Burnet in 1970 with their proposal that genetic changes leading to malignancy are common in somatic cells and that the immune system is responsible for eliminating or inactivating these potentially dangerous mutant cells.[4] This concept has now been experimentally confirmed, primarily through demonstration of the increased incidence of malignant tumors in immunodeficient mice or humans.[5] Studies have shown that severely immunocompromised mice, with deficiencies in the innate and the adaptive immune system, have a significantly increased incidence of tumors, suggesting that immunosurveillance is essential to control the gradual development of tumors.[6]
For example, recipients of solid organ transplants typically experience cancer rates similar to nontransplanted people 20–30 years older, and risk is inversely related to age, with younger recipients experiencing a far greater relative increase in risk compared with older recipients (risk increased by 15–30 times for children, but 2-fold for those transplanted >65 years).[7] Reasons for the increased risk of most cancers after transplantation are likely owing to the interplay of several factors: The organ transplanted, prior and new exposure to viral infections, total load, and duration of immunosuppression, perhaps the specific components of the immunosuppressive regimen.
However, the fact that malignant tumors also develop in patients with a fully functional immune system suggests that immunosurveillance is only a part of the process; and as a consequence, the concept of immunosurveillance has been adapted and refined over the last 15 years into a theory termed “immunoediting,”[8] a term that well describes the dual host-protecting and tumor-promoting actions of the immune system and has three phases: elimination, equilibrium, and escape. However, these are not, in fact, separate phases, but rather represent a continuum of the interplay between tumor and immune system, shifting between elimination, equilibrium, and escape depending on the state of the immune system and genuine or acquired properties of the tumor cells.
The elimination phase in the immunoediting hypothesis represents a modern view of immunosurveillance. In the equilibrium phase, although the immune system has failed in eliminating all clinically detected tumors, it can be actively and effectively engaged to keep the tumor cells in a dormant state for many years and therefore reduce the risk of metastatic spread.[9] Finally, tumor cells may escape from immune control and proliferate in an unrestricted manner, leading to clinically apparent tumors. This escape can be mediated through various mechanisms, such as reduced immune recognition, increased resistance to attack by immune cells or the development of an immunosuppressive tumor microenvironment.[2] An example of tumor immunoediting in triple-negative breast cancer (TNBC) is provided by the presence of CASP8 mutations,[10] which can abrogate the death induced by cytotoxic CD8+ T cells, and has been described as a common mechanism of immune escape in many solid tumors.[11]
There is also increasing evidence that tumors are able to create an immunosuppressive microenvironment and recruit specific immune cells that favor tumor growth and progression. Elevated levels of CD4+ regulatory T cells (Tregs) are often found in human tumors and are associated with poor prognosis. For example, Forkhead box P3 (FOXP3)+ Treg cells are crucial for the induction and maintenance of tolerance to self-antigens. While exerting their function, Treg cells can also suppress immune responses to tumor antigens.[12] Supporting this concept, in humans, FOXP3 expression in breast cancer (BC) was associated with worse distant metastases-free survival, and the risk increased with increasing FOXP3 immunostaining intensity.[13]
In a recent paper, Ahmadzadeh et al.[14] analyzed the dominant intratumoral Treg clones for antigen specificity. In Tregs from one patient’s metastatic melanoma tumor, 6 of 11 analyzed T-cell receptors (TCRs) specifically recognized the tumor, with 3 of these being among the 10 dominant FOXP3+ clonotypes. Conventional T cells from the same tumor showed 7 of 9 clones reacting to the tumor, with 3 of these being among the 10 dominant clonotypes in the intratumoral conventional T-cell subset. Furthermore, the researchers asked if tumor-specific Tregs could also be found in peripheral blood. Analyzing the TCRβ deep sequencing data from patient 3107, they found that 6 tumor-reactive Treg TCRs (including the TCR specific for the mutated ANXA1) were found in circulating Tregs. These results suggest that peripheral blood could be a source of tumor-reactive and neoantigen-specific Tregs. Overall, the results of this study demonstrate that the elevated levels of Tregs in the tumors are likely due to tumor antigen stimulation and the resulting clonal expansion. Intratumoral Tregs displayed a TCR repertoire that was distinct from conventional T cells but overlapped with circulating Tregs. Most importantly, the most dominant intratumoral Treg TCR clones showed reactivity to the tumor and patient-specific neoantigens. The identification of Treg specificity may inform the design of future TCR-based immunotherapies against cancer. In [Figure 1] are reported major functions and components of the immune system relevant for potential BC therapy.
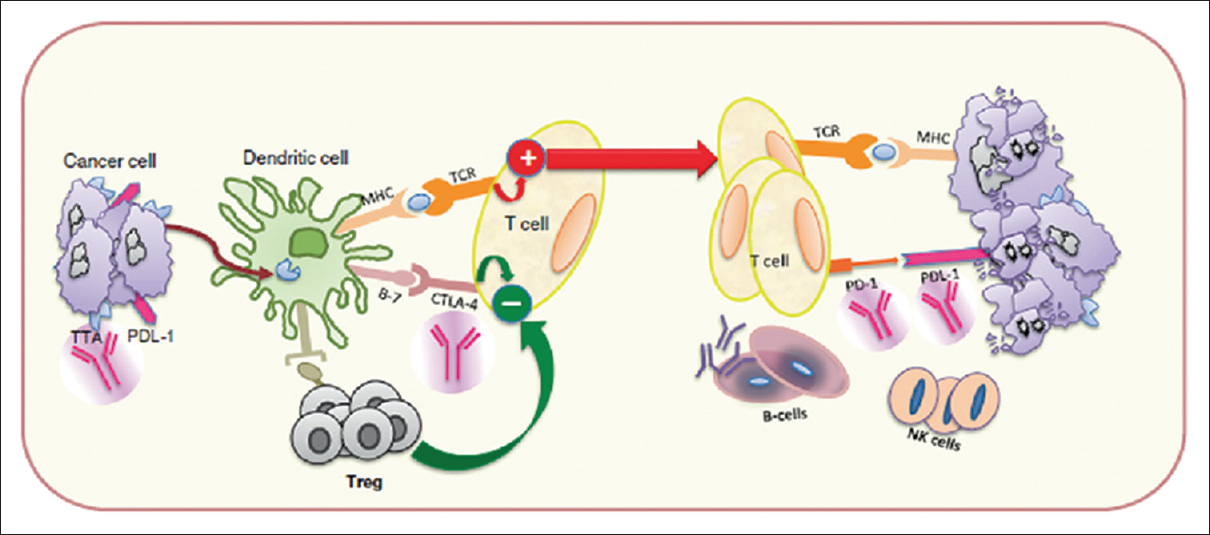 |
Figure 1: Immune system functions and components relevant to breast cancer therapy[95] Click here to view |
Immune Checkpoints
T-cells are activated by foreign antigens presented on major histocompatibility complex and the co-expression of TCR on the one hand and by a concurrent coactivation of costimulatory and/or co-inhibitory signals on the other hand [Figure 2]. The latter includes members of the CD28/B7 family and is known as “immune checkpoints.”[15],[16]
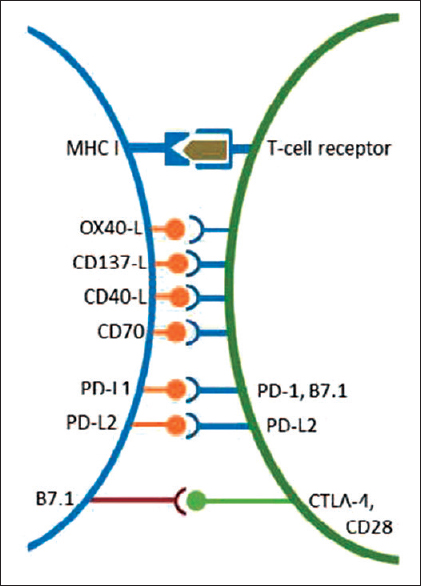 |
Figure 2: Co-stimulatory and co-inhibitory receptors expressed by T-cells (green) and target cells (rose). Reproduced with permission from Schutz F. et al. PD-1/PD-L1 pathway in Breast Cancer, Oncol Res Treat 2017 Click here to view |
Immune checkpoints are involved in T-cell tolerance as well as activation. They play a crucial role in maintaining self-tolerance and immune homeostasis under physiological conditions, thereby protecting tissues from unnecessary damage when the immune system has efficiently cleared the pathogen.[17] Even maternal immune tolerance toward the fetus is in part regulated by checkpoint inhibitors.[18]
Tumors may express immune inhibitory signals resulting in an attenuated immune reaction against the pathologic antigens.[19] Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), the programmed cell death-1 and its ligands (PD-1/PD-L1/2) axis, lymphocyte activation gene-3, and T-cell immunoglobulin mucin-3 are negative signals inhibiting T-cell immune response. In the context of tumor immunology, CTLA-4 signaling is more involved in limiting the initiation of a T-cell response in the lymph nodes, while PD-1 features more prominently later on in the process and serves to limit T-cell activity in the tumor microenvironment.[20] After TCR engagement, CTLA-4 is upregulated to attenuate T-cell responses and prevent expansion of autoreactive T cells, primarily during the priming phase within the lymph nodes. Anti-CTLA antibodies such as ipilimumab and tremelimumab were both tested in solid tumors including BC with limited efficacy.[21],[22]
Programmed Cell Death-1/programmed Cell Death Ligand-1 Pathway
PD-1 is an inhibitory immune checkpoint inhibitor (ICI) which is expressed on the surface of T cells, B cells, natural killer (NK) T cells, T-cell lysis, and causes induction of tolerance to antigens.[23],[24],[25]In vitro blockade of PD-1 with monoclonal antibodies (mAbs) led to a 2-fold increase in cytokine production.[26] However, thein vivo activity also depends on T-cell motility as well as the duration of the interaction with antigen-presenting cells and target cells.[27] When T cells have been activated by their TCR, PD-1 is expressed simultaneously to offer the attacked cell a way of escaping the immune reaction. PD-1 decreases once the immune response has eliminated the pathologic antigen.[28]
In solid tumors, the PD-1/PD-L1 inhibitory pathway cause inactivation of the immune system by increasing the expression of PD-L1 on the tumor cell surface.[29] PD-L1 expression has been associated with large tumor size, high grade, high proliferation, estrogen receptor (ER)-negative status, and human epidermal growth factor receptor-2 (HER2)-positive status,[30] and it is inversely correlated with survival in ovarian[31] and BC.[32] PD-L1 is expressed in 20% of TNBCs.[33] This indicates that although antitumor immunity is elicited against many solid tumors, it is counterbalanced by immunosuppressive factors. It was shownin vivo that PD-L1 increases tumorigenesis and invasiveness and makes tumor cells less susceptible to specific CD8+ T-cells.[34] The goal of ICIs such as anti-CTLA-4 and anti-PD-1/anti-PD-L1 is to “release the brakes” and enhance T-cell activation by blocking negative pathways.
Furthermore, myeloid-derived suppressor cells (MDSC) play a leading role in immunosuppression in various cancer types. Accumulating evidence in recent years have even highlighted them as a major driver of an immunosuppressive tumor microenvironment.[35] The research shows that although ICI may prove to be effective, therapeutic resistance occurs in the majority of patients, leading to tumor progression.[36] This occurs due to the immunosuppressive tumor microenvironment represented by several immunosuppressive factors and cells, including MDSC. Importantly, the efficacy of cancer immunotherapy has been reported to be negatively correlated with an increased MDSC frequency and function.[37] Therefore, MDSC could be a promising target in cancer immunotherapy, especially in combination with ICI.
Prognostic Value of Immune-Related Gene Signatures
In past years, gene expression profiling has been used in an effort to more precisely define BC taxonomy and identify prognostic and predictive signatures.[38] The common denominator between the majority of the “first generation” signatures is their overall capacity to detect subtle differences in the cell cycle and proliferation. For this reason, they have not been found to be prognostic in the triple-negative or HER2+ subtypes since these tumors are by “nature” highly proliferative. Several investigators have tried to overcome the limitations of these “ first generation” signatures by focusing on the BC microenvironment or immune response (or both) to define promising “second generation” prognostic signatures.[39] Unsupervised gene expression profiling of cancer-associated stroma revealed a signature enriched for CD8+ T-cell responses that was predictive of good prognosis.[40] An immune response module, the STAT1 module, has been shown to be associated with survival in patients with TNBC and HER2+ BC,[41],[42] and in the same BC subtypes, the overexpression of immune-related genes was able to identify subgroups of patients with a better prognosis.[43],[44] Similarly, in other studies, the high expression of B-cell and immunoglobulin-based metagenes is associated with a low risk of developing distant metastases in patients with untreated ER-negative breast tumors,[42],[45] whereas in patients with untreated ER-negative HER2-positive BC, elevated expression of the STAT1-related and T-cell-related metagenes is associated with a low risk of distant metastasis.[46] These studies suggest that effective engagement of the immune system, although insufficient to eliminate the tumor, can help to reduce the risk of tumor spreading, or maintain tumor dormancy.[9]
The Role of the Lymphocytic Infiltrate in Breast Cancer
The presence of tumor-infiltrating lymphocytes (TILs) is observed in some breast tumors and has been reported to be a good prognostic feature for certain types of BCs,[43],[47] particularly for ER-negative tumors and HER2-positive subtypes.[48] Furthermore, TILs have been negatively correlated with the patient’s age at diagnosis.[49] More recently, the nature of TILs has been better characterized. Ruffell et al.[50] have reported that TILs are composed mainly of CD3+/CD56− T cells but that a minority consisted of NK cells or CD20+ cells. The majority of CD3+ cells were either CD4+ or CD8+ T cells. Interestingly, CD8+ cells did not express granzyme B at baseline, which means that they did present inactivation status, but they did express granzyme B after neoadjuvant chemotherapy in one-third of the patients. Finally, a minority of TILs presented T and NK-cell features.[50] The genomic characteristics of TIL+ tumors are important to understand which molecular mechanisms lead to lymphocyte infiltration. Genomic instability may promote anti-tumor immune response through tumor-associated antigens (TAAs). Some mechanisms of chemokine release by the tumor have been described and correlated with lymphocyte attraction. TILs have been associated with CXCL9 and CXCL13 expression by the tumor.[48] TIL + tumors present a specific methylation pattern on immune-related genes, including CCL5,[51] and a cluster of chemokines is lost in a subset of BC.[52]
In TNBC and HER2-positive BC, the association between the presence of TILs or expression of immune markers and the likelihood of achieving a pathologic complete response (pCR) after neoadjuvant chemotherapy is consistent and strong. A high level of expression of immune markers is associated with different immune cell types and functions and has been associated with benefit from chemotherapy in TNBCs.[30],[46],[53] This association has also been confirmed by evaluation of the TIL density.[48],[54] Overall, these data suggest that the immune system collaborates with the action of chemotherapy in TNBCs, as suggested by data from preclinical studies.[55],[56] However, whether drug-specific immunomodulation properties,[56] such as inducing immunogenic tumor cell death (postulated for anthracyclines) or engaging different immune effector mechanisms, are associated with different clinical outcomes is unknown and is currently an active area of investigation. Interestingly, assessments of the immune microenvironment after neoadjuvant chemotherapy in patients with TNBC with residual disease has shown that the immune microenvironment can be turned from “cold” (containing few TILs) to “hot” (higher TIL presence) in some patients.[57] Tumors that remain or become “cold” after chemotherapy have a higher risk of relapse compared with tumors that remain or become “hot.”[57] These data also support the concept of chemotherapeutic agents having immunomodulatory activity, and thus, acting as an immunological stimulant in the tumor microenvironment to induce antitumor immunity.[57] Whether the immune system has different prognostic and predictive roles in different molecular subtypes of TNBCs has not been defined yet.
Overall, considering that, in TNBCs, a “hot” immune microenvironment is associated with a better prognosis and a higher likelihood of benefit from chemotherapy, it should not be surprising that many investigators have identified a strong association between high levels of immune markers or TILs and a low risk of relapse and/or death in patients with early-stage TNBC treated with systemic chemotherapy.[58] These results suggest that, in TNBC, the risk of recurrence in the early disease setting can be effectively defined by adopting the appropriate immune markers for risk stratification. These results also distinguish a subgroup of patients with TNBC characterized by a “cold” immune microenvironment that has a high risk of relapse, despite treatment, and a low likelihood of benefit from cytotoxic chemotherapy.[46] Evidence for the clinical utility of TIL evaluation, however, is still scarce, in part because TIL assessment lacks sufficient standardization; however, efforts to improve consistency and reproducibility are underway.[59]
Immunogenicity of Breast Cancer
BC has not been traditionally considered immunogenic, as opposed to melanoma and renal cell carcinoma, which have been traditionally considered more responsive to immunotherapies. Moreover, the tumor microenvironment in BCs releases immune-suppressive factors that make the antigen presentation difficult and that have a negative impact on the immune response.[60] Furthermore, by blocking endogenous immune checkpoints that normally terminate immune responses after antigen activation, it is possible to evade immune destruction.
However, it seems that, despite a weak influence on primary tumor growth, the immune system may be effective in preventing BC metastases.[61],[62] It seems that any tumor could be immunogenic with appropriate immune activation. In a neoadjuvant clinical trial (Trial of Principle study) in which patients with ER-negative BCs were treated with anthracycline monotherapy, high immune module scores were associated with sensitivity to anthracyclines.[53] The immune system seems also to be pivotal in determining the response to mAbs and tyrosine kinase inhibitors, and some evidence indicates a possible role in the response to endocrine treatment. Antibody-dependent cellular cytotoxicity has long been implicated as one of the mechanisms of action for trastuzumab.[63],[64] Therefore, complete tumor response after molecular targeted therapies requires a functioning immune system, pointing the way toward radically new combination therapies with a targeted and immune approach.[65] The mAbs against antigen tumor target or immune-regulatory molecules, cell-based therapies including adoptive transfer of ex vivo-activated T cells and NK cells, or blockade of Treg cells could be useful to amplify the anti-tumor response.
Tumor Mutational Burden and Mutational Signatures in Breast Cancer
The use of immunotherapy is exponentially increasing in the treatment of patients with advanced solid tumors. However, the response rates vary significantly between different tumor types and even within the same tumor type (e.g., in lung cancer approximately 1 in 4 patients respond to immunotherapy). In order to better identify patients that will respond to immunotherapy, several markers have been proposed. Tumor mutational burden (TMB) has emerged more recently as a quantitative marker that can help predict responses to immunotherapies across different cancers, including melanoma, lung cancer, and bladder cancer and BC.[66] TMB is a measure of the overall number of somatic protein-coding mutations occurring in the tumor specimen. Bonta et al.[67] analyzed 54 patients with solid tumors treated with immunotherapy for which they had genomic sequencing (FoundationOne). There were 39 lung cases and 15 nonlung (gastrointestinal, genitourinary, sarcoma, and breast). Among patients with known TMB, 60% (18/30) had a favorable response (stable disease or response to therapy). Higher TMB values were correlated with increased probability of a favorable response. In their study, a TMB cutoff value of 8 mutations (mut)/megabase (MB) yielded a sensitivity of 95% and a specificity of 58% for predicting favorable response. In 2018, ASCO annual meeting, Barroso-Sousa et al.[68] evaluated mutational load across BCs. Samples were classified as having high TMB if they had >10 mut/MB. They included 3689 samples for the analysis. The median TMB was 1.55 mut/MB. TMB significantly varied according to histology (ductal > lobular, P = 4.6 × 10–13), tumor subtype (HR-/HER2+ > TNBC > HR+/HER2+ > HR+/HER2-, P < 0.05), staging (metastatic > primary, P = 2.2 × 10–16) and site of metastasis (higher soft tissue, and lowest lung, P < 0.05). They found a total of 70 (~2%) hypermutated tumors (62.8% metastatic vs. 37.2% primary samples). Mutational signature analysis of the hypermutated samples showed the presence of dominant APOBEC (77.1%), homologous recombination (HR; 2.9%), defective DNA mismatch repair (MMR; 18.6%), and POLE hypermutation (1.4%) signatures. Median TMB was higher for samples with POLE and HR signature, followed by those with MMR and APOBEC (93.1, 38.7, 14.6, and 12.4 mut/MB, respectively). Among hypermutated tumors, eight samples had somatic mutation in the POLE gene but only the case with POLE signature high had a characterized POLE driver mutation. In addition, 80% of hypermutated tumors with APOBEC signature had PIK3CA mutations versus 31% of hypermutated tumors with other signatures (P = 0.0005). In another study, Xu et al.[69] aimed to predict the level of TMB in patients with BC by the expression of ER, PR, HER-2, and Ki-67, thereby anticipating the prognosis of patients and the possible response to immunotherapy. HER-2 expression positivity was significantly associated with TMB (HER-2 positive vs. HER-2 negative, odds ratio [OR] = 34.81, 95% confidence interval [CI]: 3.711–821.689, P = 0.0065). Furthermore, higher TMB was distributed in the patients who were Ki-67 expression positive (>14%) than those who were Ki-67 expression negative (≤14%) (OR = 0.217, 95% CI: 0.054–0.806, P = 0.0242). However, no significant differences of TMB were found between ER-positive group and ER-negative group (OR = 3.133, 95% CI: 0.124–127.687, P = 0.4954) and between PR-positive group and PR-negative group (OR = 1.702, 95% CI: 0.162–20.335, P = 0.6492). In a multivariate analysis, high TMB (>5.56) was an independent predictive factor for decreased disease free survival (DFS) (adjusted hazard ratio [HR], 5.594; 95% CI: 1.694–18.473; P = 0.005). These results suggest that the level of TMB value can be predicted based on the expression levels of ER, PR, HER-2, and Ki-67, which may indicate the prognostic and predictive value of immunotherapy in patients with BC.
Immunotherapy for Triple-Negative and Human Epidermal Growth Factor Receptor 2-Positive Breast Cancers
Immunotherapy with checkpoint inhibitors has made a significant impact in the treatment of melanoma, renal cell carcinoma, and non-small cell lung cancer (NSCLC) in recent years.[70],[71],[72],[73] New agents such as nivolumab and pembrolizumab (a fully human IgG4 programmed death 1 [PD-1] ICI antibody) selectively blocks the interaction of the PD-1 receptor with its two known programmed death ligands, PD-L1, and PD-L2, disrupting the negative signal that regulates T-cell activation and proliferation.[74] Anti-PD-L1, such as atezolizumab is the first FDA approved PD-L1 blocker and has been used as first-line treatment for cisplatin-resistant metastatic urothelial carcinoma, metastatic NSCLC. The variety of clinical trials is under progress for colorectal cancer, bladder cancer, renal cell carcinoma, head and neck cancer, and GI malignancies. Durvalumab (MEDI4736), another PD-L1 blocker, binds to PD-1 and CD80, is approved in unresectable NCSLC and locally advanced or metastatic urothelial carcinoma. Avelumab is also another PD-L1 blocking antibody indicated for the treatment of adults and pediatric patients 12 years and older with metastatic Merkel cell carcinoma.
As mentioned before, there is preliminary evidence of positive correlation between high mutational burden of tumors and clinical benefit from immunotherapy strategies (i.e., checkpoint inhibitors anti-CTLA-4 and anti-PD-1 antibodies), with remarkable effects seen with tumors displaying the highest rates of mutations such as melanoma.[75],[76] This is also illustrated by the antitumoral immunologic response to anti-PD-1 antibody in patients with colorectal cancer and increased mutational burden secondary to mismatch repair deficiency.[77]
As discussed above, in recent years, the better knowledge of BC biology has provided an opportunity to develop some types of immunotherapy to overcome the relative nonimmunogenic property of BC and improve immune response. TNBCs have a higher number of TILs[78] and higher PD-L1 protein[79],[80] or mRNA[33],[58] expression compared with other BC subtypes. PD-L1 expression is significantly associated with the presence of TILs,[80] which suggests that the most common mechanism of regulation of PD-L1 expression in TNBC is regulatory feedback (acquired resistance) to immune engagement. An extremely heterogeneous pattern of immune infiltration, however, has been described among TNBC subtypes.[81] A significant association has also been found between PD-L1 mRNA expression and the presence of PD-L1 copy-number alterations, with basal-like BC having the highest frequency of PD-L1 gains/amplifications (17%).[30] In addition, the loss of PTEN expression in TNBCs is associated with PD-L1 overexpression,[33] confirming an association between increased PI3K signaling and the presence of PD-L1.
The finding that a population of TNBC is immunogenic and actively engaged by the immune system provides a strong rationale for testing immunotherapies in this type of BC. The potential importance of immune checkpoint guided therapy in TNBC is underscored by recent reports. Several Phase I trials with immune-checkpoint inhibitors in patients with advanced-stage TNBC have been reported.[82] In one of them, 188 patients with advanced-stage TNBC positive for PD-L1 expression were treated with the anti-PD-1 mAb pembrolizumab with an overall response rate (ORR) of 18.5% (five out of 27 patients). Seven additional patients had stable-disease. Of the screened patients, ≥1% PD-L1 expression was detected using IHC labeling of stromal or tumor cells in archival specimens from 58% of patients with the 22C3 antibody. Avelumab, an anti-PD-L1 IgG1 antibody, showed modest anti-tumor activity among 57 patients with TNBC with only 5 partial responses (PR) observed (8.8%; 95% CI: 2.9, 19.3).[83] In patients with TNBC who had PDL1+ immune cells within the tumor, 44.4% (4 of 9) had PR, compared with 2.6% (1 of 39) PD-L1-negative immune cells. In another Phase I study,[84] selectively enrolled cohort of patients with PD-L1-positive metastatic TNBC patients received MPDL3280A, a human anti-PD-L1 mAb, 15 or 20 mg/kg IV every 3 weeks for up to 1 year. The ORR was 33%, including 1 complete response and 2 PRs. Responders included patients with visceral metastases at baseline. At the time of the clinical data cutoff, all responses occurred within 6 weeks of the first dosing of MPDL3280A, and all of the responses were ongoing. The median duration of response had not been reached (range: 18 to 56+ weeks). Different trials are ongoing to establish the safety and clinical activity of ICIs in both PD-L1-positive and PD-L1-negative TNBCs, alone or in combination with chemotherapies [Table 1].
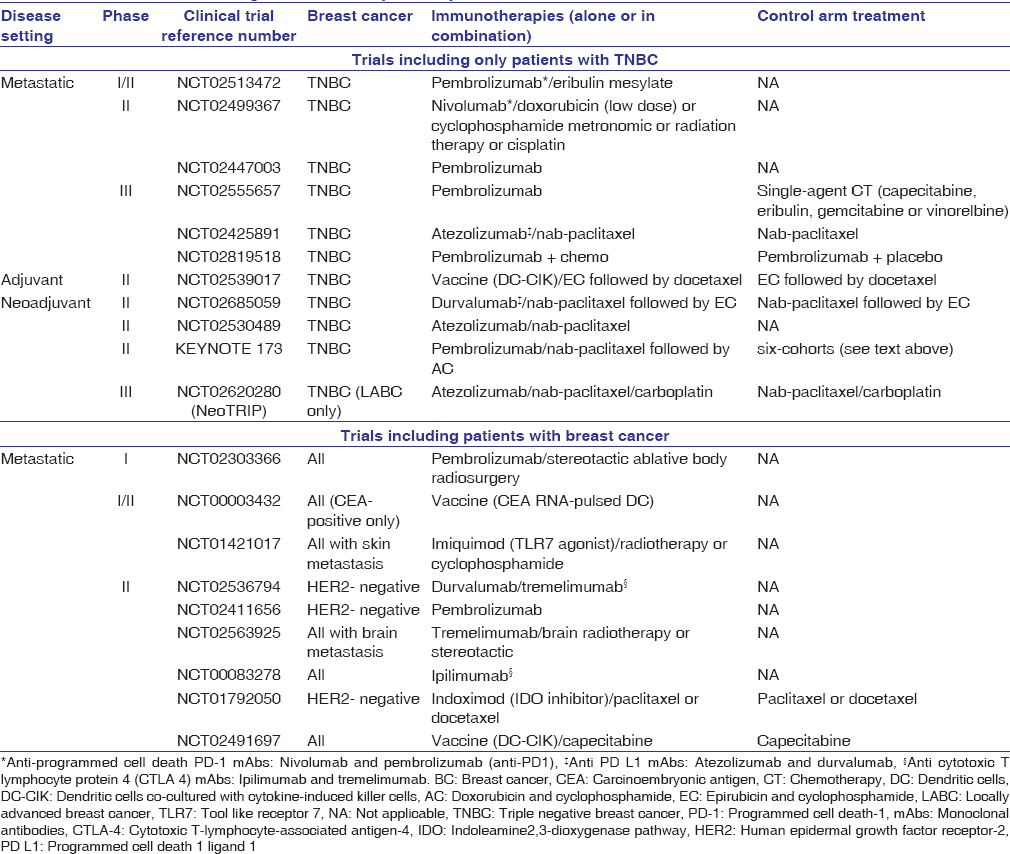 |
Table 1: Clinical trials testing immunotherapies in patients with breast cancer Click here to view |
In light of the promising preliminary results obtained with immune-checkpoint inhibitors, their expected curative potential[85] and their beneficial safety profile, these agents are already being assessed for the treatment of patients with early-stage TNBC. Three trials in patients with Stage I–III TNBC are currently ongoing to evaluate the potential activity of immune-checkpoint inhibitors in combination with chemotherapy in the neoadjuvant setting. In the Phase III trial NeoTRIPaPDL1 (NCT02620280), patients with locally advanced TNBC will be randomly assigned to receive nab-paclitaxel and carboplatin with or without a PD-L1-inhibitor (atezolizumab). Notably, the primary endpoint will be event-free survival. A Phase II trial will evaluate atezolizumab in combination with nab-paclitaxel (NCT02530489). Finally, a Phase I/II trial will test the safety and efficacy of durvalumab, another anti-PD-L1 antibody, in combination with weekly nab-paclitaxel followed by dose-dense chemotherapy containing cyclophosphamide and doxorubicin (NCT02489448).
Another interesting immune molecule is CTLA-4 (CD152), which is similar to PD-1, but its immune inhibitory signals are different. CTLA-4 knockout mice display early lethality, unlike PD-1 knockouts, which demonstrate late-onset and organ-specific autoimmunity. Anti-CTLA4 mAb treatment has shown robust tumor responses in Phase III trials, but with considerable adverse events.[86] Still, combining anti-CTLA-4 mAb with trastuzumab has shown synergy in preclinical mouse models.[87] Hence, immune-therapeutics that augment CD8 T-cell anti-tumor activity – such as anti-PD1 and anti-CTLA4 mAbs – given in combination with trastuzumab in patients with HER2+ BC may improve outcome by enhancing host immunity.[65],[88],[89] Given this evidence, the evaluation of baseline immune response and the identification of surrogate markers of immune system activation could be helpful in the management of BC to identify patients who may benefit from these combination therapies, even eliminating the need for combination cytotoxic chemotherapy.
It is important to note that a subset of patients treated with ICIs experience an accelerated tumor growth rate compared with pretreatment kinetics, known as hyperprogression. Kanjanapan et al.[90] explored the relationship between hyperprogressive disease (HPD), treatment-related toxicity, and clinical factors. They observed a 7% rate of HPD within a range of solid tumors treated with ICIs, comparable to other reports. There was no association between HPD and clinically significant adverse events, age, tumor type, or type of therapy. We need further studies to identify predictors of HPD.
Immunotherapy for Estrogen Receptor-Positive Breast Cancer
The immunotherapy has so far shown more limited responses in ER-positive BCs compared to TNBCs that tends to overexpress PDL1 as outlined above. ER + BCs may be less immunogenic, with inadequate activation of immune effector cells and/or other adaptations in the tumor microenvironments that suppress antigenicity and/or suppress activation of the adaptive or innate immune systems. Studies to determine how to reestablish immune-based host elimination of tumors offer potential, particularly for the eradication of the many small foci of growth arrested but surviving cells that remain during treatment with endocrine therapies and are the source of distant recurrences in ER + BCs.
Immunotherapy for Inflammatory Breast Cancer
The role of immune infiltrate and immune checkpoints was also investigated in relation with genomic abnormalities in IBC samples.[91] The pathological examination of 20 IBC tissue samples identified a subset of IBC tumors associated with infiltration of immune cells. IHC staining identified the majority of infiltrating cell populations as CD8+ cytotoxic T cells, and high levels of CD8+ infiltration was observed in 5/12 tumors. In order to explore the possible role of PD-L1 in IBC, the investigators performed IHC staining of IBC tissues. The evaluation of PD-L1 staining demonstrated low-intensity tumor cell staining in 3/12 tumors studied and high-intensity tumor cell staining in 1/12 tumors. PD-L1 mRNA expression has been reported to as high as 38% among patients with IBC, which is higher than non-IBC (28%) and correlates positively with pCR.[92] Notably, somatic mutation rates were significantly higher in high infiltration versus low infiltration tumors (P < 0.05).[91] The authors speculated that this correlation between somatic mutation rate and immune cell infiltration might be related to the exposure of tumor neo-antigens to the immune system. A Phase II clinical trial for patients with metastatic IBC assessing the efficacy of anti-PD-1 inhibitor monoclonal antibody (pembrolizumab) is under development (NCT02411656).
Maintenance Immunotherapy in Patients With Metastatic Breast Cancer Who Have a Clinical Benefit With Chemotherapy
Recchia Fet al.[93] investigated the effect of maintenance immunotherapy with the aim of prolonging progression-free survival (PFS) and overall survival (OS), through immune-mediated mechanisms, patients with hormone-resistant MBC who had not progressed with chemotherapy. From 1996 to 2009, 74 patients with MBC were entered into the study and received the following maintenance immunotherapy regimen: Interleukin-2 (IL-2), (1.8 M UI), and oral retinoic acid (0.5 mg/kg), 5 days/week, 3 weeks/month for 1 year. Therapy was continued, with intermittent schedule until progression. The median age was 55 years (range 31–75); 30% of patients were premenopausal; 60% were ER + and had progressed after 2 or more lines of hormonal therapy. The 74 patients had received 368 courses of chemotherapy regimens (median of 6 courses each patient). Thirty-six patients had received, also, high-dose chemotherapy with peripheral blood progenitor cell transplantation. After a median follow-up of 100 months (range 96–200), each patient had received a median of 8 courses of immunotherapy (a total of 924 courses delivered). No WHO Grade 3 or 4 toxicity was observed; Grade 2 cutaneous toxicity and autoimmune reactions occurred in 19% and 16% of patients, respectively. Statistically significant improvements were observed in the number of lymphocytes (P < 0.001), natural-killer cell count (P < 0.001), and the CD4+/CD8+ ratio (P < 0.01). A 10-year PFS and OS were 25% and 31%, respectively. Although these data needs to be matured in BC field, this approach has already been proven significant in improving PFS and OS in Stage III NSCLC patients. In February 2018, FDA approved durvalumab as a maintenance therapy for patients with unresectable Stage III non-small cell lung cancer whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.[94]
Vaccine-Based Therapies for Breast Cancer
Vaccines constitute an active and specific immunotherapy designed to stimulate the intrinsic anti-tumor immune response by presenting TAAs expressed on normal tissues that are overexpressed on tumor cells. Malignant cells can express both normal self-antigens and specific TAAs that arise from genetic mutations or epigenetic changes or both, recognized by the immune response through either their loss or de novo aberrant expression. Many TAAs (including MUC1, HER-2, CEA, hTER, and WT1) have been identified and been shown to be specifically recognized by T cells.[95] The induction of strong immunity by cancer vaccines is expected to lead to the establishment of immunological memory, thereby preventing tumor recurrence.
CAR T-cell therapy is an innovative form of immunotherapy wherein autologous T cells are genetically modified to express chimeric receptors encoding an antigen-specific single-chain variable fragment and various costimulatory molecules [Figure 3]. CARs are recombinant receptors that typically target native cell surface antigens.[96] Unlike the physiological TCR, which engages human leukocyte antigen (HLA)-peptide complexes, CARs engage molecules do not require peptide processing or HLA expression to be recognized. Therefore, on administration, these modified T cells traffic to, and recognize, cancer cells in an HLA-independent manner.[96]
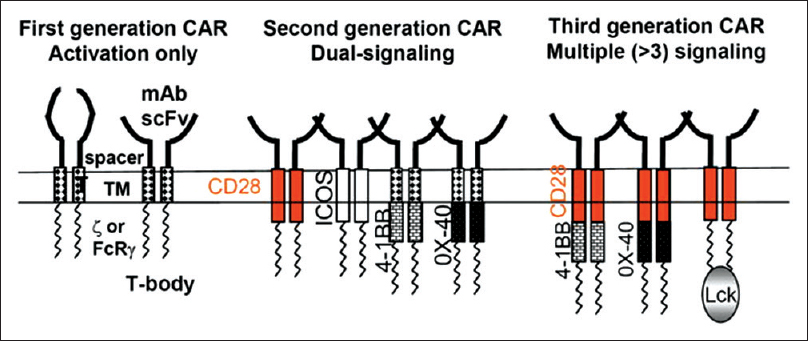 |
Figure 3: The general architecture of a chimeric antigen receptor consists of a single-chain variable fragment derived against a predetermined tumor-associated antigen followed by a CD3ζ domain required for provision of signal 1 and T-cell activation upon antigen recognitionBayraktar Click here to view |
HER2 and mesothelin are two TAAs currently under investigation. Amplification of HER2 oncogene leads to uncontrolled cell proliferation and occurs in approximately 20% of BCs.[97] Globerson-Levin et al.[98] generated a HER2-specific, second-generation CAR containing CD28, and fragment crystallizable receptor signaling domains and tested its efficacy in a syngeneic mouse mammary tumor model. Transduced T cells exhibited potent cytotoxic capacity and cytokine secretion on antigen recognition. In addition, repeated injections of HER2-directed CAR T cells eliminated spontaneous HER2-positive tumors and enhanced survival in transgenic mice. Mesothelin is a glycoprotein expressed on a broad range of solid tumors, with limited expression on normal tissues.[99] Mesothelin expression has been shown to be enriched in TNBC and is associated with poor outcomes.[100] Patients with TNBC are not suitable for targeted therapy or hormone therapy, so adoptive transfer of mesothelin-specific CAR T cells offers an alternative option. Tchou et al.[101] engineered mesothelin-specific CAR T cells and reported a cytolytic capacity against primary breast tumor cells in vitro. However,in vivo antitumor activity was not evaluated in this study.
A major therapeutic challenge to therapy in BC is acquired resistance that results from antigen escape. For instance, under selective pressure, HER2 can undergo proteolysis to cleave the extracellular domain without compromising kinase activity. One approach to overcome this limitation is to use a dual-targeting CAR system, in which engineered T cells coexpress two CARs that recognize two distinct antigens. Redirected T cells can be activated in the presence of either antigen to mitigate antigen-loss escape.[102] Alternatively, CAR T cells can be modified to secrete inflammatory cytokines, such as IL-12, or costimulatory ligands, such as 4-1BB ligand, to stimulate an endogenous immune response against tumor cells via epitope spreading.[103]
The Promise of Immunotherapy in Combination With Chemotherapy in Breast Cancer
Immunotherapies and cancer vaccines are more effective when given in combination with standard cancer treatments, which appear to increase their effectiveness by initiating tumor-specific immunity by increasing antigen presentation, MHC upregulation, increase T cell function, increased release of pro-inflammatory cytokines and cross-presentation through dendritic cells.[104],[105] The elimination of Treg cells and reduction of MDSC potentially provides another basis for the synergistic effect between cancer vaccines and chemotherapy.[105] Existing clinical data regarding chemoimmunotherapy from patients with other solid tumors have shown that patients who receive atezolizumab plus chemotherapy[106] or pembrolizumab plus chemotherapy[107] have improved OS and DFS compared to patients who receive standard chemotherapy alone.
Nab-paclitaxel as partner with ICI is particularly appealing to researchers trying to improve the chemoimmunotherapy trial results for several reasons. First and foremost, steroids which may downregulate antigen presentation are not a required premedication before nab-paclitaxel infusion;[108] it has a well-characterized and acceptable adverse event profile and has a distinct pharmacokinetics profile with higher accumulation in tumor microenvironment versus paclitaxel.
Recently, the primary results from the Phase III IMpassion130 trial[109] establish the benefit of adding ICI to nab-paclitaxel in patients with TNBC. In this Phase III trial, patients with untreated metastatic TNBC were randomly assigned to receive atezolizumab plus nab-paclitaxel or placebo plus nab-paclitaxel. In the intention-to-treat analysis, the median PFS was 7.2 months with atezolizumab plus nab-paclitaxel, as compared with 5.5 months with placebo plus nab-paclitaxel (HR: 0.80; 95% CI, 0.69–0.92; P = 0.002); among patients with PD-L1–positive tumors, the median PFS was 7.5 and 5.0 months, respectively (HR: 0.62; 95% CI, 0.49–0.78; P < 0.001). In the intention-to-treat analysis, the median OS was 21.3 months with atezolizumab plus nab-paclitaxel and 17.6 months with placebo plus nab-paclitaxel (HR: 0.84; 95% CI, 0.69–1.02; P = 0.08); among patients with PD-L1-positive tumors, the median OS was 25.0 and 15.5 months, respectively (HR: 0.62; 95% CI, 0.45–0.86). No new adverse effects were identified.
Another Phase II trial[110] results also showed the safety and improved the efficacy of concurrent administration of durvalumab (10 mg/kg) with weekly nab-paclitaxel (100 mg/m2) ×12 followed by dose-dense AC (doxorubicin and cyclophosphamide) ×4 as neoadjuvant therapy for Stage I-III TNBC. The primary efficacy endpoint was pCR. Seven of the 12 patients achieved pCR. Eight patients (25%) experienced Grade 3 adverse events including 3 patients with neutropenia (1 neutropenic fever), and one patient each with fatigue, dyspnea, line infection, transaminitis, hypertension/skin rash. No perioperative adverse events were seen.
The addition of pembrolizumab to neoadjuvant chemotherapy demonstrated promising antitumor activity and exhibited a manageable toxicity profile in a cohort of patients with early-stage TNBC, according to results of the KEYNOTE-173 trial[111] presented at 2018 San Antonio BC Symposium. All patients received a single dose of pembrolizumab 200 mg on day 1 of the first cycle. In the second to fifth cycles, patients received pembrolizumab 200 mg plus one of the six chemotherapy regimens: Nab-paclitaxel 125 mg/m2 once weekly (cohort A); nab-paclitaxel 100 mg/m2 once weekly plus carboplatin area under the curve 6 every 3 weeks (cohort B); nab-paclitaxel 125 mg/m2 once weekly plus carboplatin area under the curve 5 every 3 weeks (cohort C); nab-paclitaxel 125 mg/m2 once weekly plus carboplatin area under the curve 2 once weekly (cohort D); paclitaxel 80 mg/m2 once weekly plus carboplatin area under the curve 5 every 3 weeks (cohort E); and paclitaxel 80 mg/m2 once weekly plus carboplatin area under the curve 2 once weekly. In the sixth to ninth cycles, all patients received doxorubicin 60 mg/m2 every 3 weeks and cyclophosphamide 600 mg/m2 every 3 weeks plus pembrolizumab 200 mg every 3 weeks. Researchers reported ORRs of 100% (90% CI, 74–100) in cohorts B and C, 90% (90% CI, 61–100) in cohorts D and F, 80% (90% CI, 49–96) in cohort A, and 70% (90% CI, 39–91) in group E. Eighteen patients (30%) experienced immune-related adverse events, the most common of which were Grade 2 hypothyroidism (n = 4), Grade 1 hyperthyroidism (n = 3), Grade 3 colitis (n = 2), and Grade 3 rash (n = 2). Sixty percent (90% CI, 30–85) of all patients achieved pCR. The findings support the ongoing Phase III KEYNOTE-522 trial, which is comparing the combination with placebo in this patient population. Importantly, these ongoing trials will answer the question whether the correlation between pCR with chemo-immunotherapy and long-term outcomes is as clear as the correlation between pCR and chemotherapy in this patient population.
Conclusion
Immunomodulation seems to be a promising strategy in solid tumors. High immunogenicity has been described in BC subtypes with a high proliferation index (TNBC and HER2). Immune checkpoints are one of the major mechanisms of immune escape. Expression of PD-L1 on tumor cells leads to lower activity of CD8+ T-cells. Antibodies against PD-1 or PD-L1 are being investigated in clinical trials. First results are promising but only a subset of patients (20%) respond to immune checkpoint inhibitory treatment. Predictive markers are urgently needed to select those patients with the best chance for an effective treatment.[112] One possible avenue is immuno-molecular therapy, which integrates immune and molecular features to devise novel combinatorial approaches based on targeting intracellular molecular alterations and modulating the immune response.[113]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. |
Tripathi S, Guleria I. Role of PD1/PDL1 pathway, and TH17 and treg cells in maternal tolerance to the fetus. Biomed J 2015;38:25-31.
 [PUBMED] [Full text] |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. | |
| 25. | |
| 26. | |
| 27. | |
| 28. | |
| 29. | |
| 30. | |
| 31. | |
| 32. | |
| 33. | |
| 34. | |
| 35. | |
| 36. | |
| 37. | |
| 38. | |
| 39. | |
| 40. | |
| 41. | |
| 42. | |
| 43. | |
| 44. | |
| 45. | |
| 46. | |
| 47. | |
| 48. |
Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33:983-91.
 |
| 49. | |
| 50. | |
| 51. | |
| 52. | |
| 53. | |
| 54. | |
| 55. | |
| 56. | |
| 57. | |
| 58. | |
| 59. | |
| 60. | |
| 61. | |
| 62. | |
| 63. | |
| 64. | |
| 65. | |
| 66. | |
| 67. | |
| 68. | |
| 69. | |
| 70. | |
| 71. | |
| 72. | |
| 73. | |
| 74. | |
| 75. | |
| 76. | |
| 77. | |
| 78. |
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31:860-7.
 |
| 79. | |
| 80. | |
| 81. | |
| 82. | |
| 83. | |
| 84. | |
| 85. | |
| 86. | |
| 87. | |
| 88. | |
| 89. | |
| 90. | |
| 91. | |
| 92. | |
| 93. | |
| 94. | |
| 95. | |
| 96. | |
| 97. | |
| 98. | |
| 99. | |
| 100. | |
| 101. | |
| 102. | |
| 103. | |
| 104. | |
| 105. | |
| 106. | |
| 107. | |
| 108. | |
| 109. | |
| 110. | |
| 111. |
Schmid P, Cortes J, Bergh JC, Pusztai L, Denkert C, Verma S. KEYNOTE-522: Phase III study of pembrolizumab (pembro) plus chemotherapy (chemo) vs. placebo plus chemo as neoadjuvant therapy followed by pembro vs. placebo as adjuvant therapy for triple-negative breast cancer (TNBC). Asco Annual Meeting (Chicago), J of Clinical Oncology 2018;36 suppl 15: TPS602.
 |
| 112. | |
| 113. |
