Qiao Yi Chen, Max Costa
Department of Environmental Medicine, New York University School of Medicine, NY, USA
| Date of Submission | 17-Sep-2017 |
| Date of Acceptance | 28-Dec-2017 |
| Date of Web Publication | 05-Apr-2018 |
Correspondence Address:
Dr. Max Costa
Department of Environmental Medicine, New York University School of Medicine, 57 Old Forge, Tuxedo, New York 10987, NY
USA
Source of Support: None, Conflict of Interest: None
Abstract
In recent years, research efforts have been centered on the functional roles of special AT-rich sequence-binding protein (SATB2) in cancer development. Existing studies differ in the types of tumor tissues and cell lines used, resulting in mixed results, which hinder the clear understanding of whether SATB2 acts as a tumor suppressor or promoter. Literature search for this review consisted of a basic search on PubMed using keywords “SATB2” and “special AT-rich sequence-binding protein 2.” Each article was then selected for further examination based on relevance of the title. In consideration to possible missing data from a primary PubMed search, after coding for relevant information, articles listed in the references section were filtered for further review. The current literature suggests that SATB2 can act both as a tumor suppressor and as a promoter since it can be regulated by multiple factors and is able to target different downstream genes in various types of cancer cell lines as well as tissues. Future studies should focus on its contradictory roles in different types of tumors. This paper provides a comprehensive review of currently available research on the role of SATB2 in different cancer cells and tissues and may provide some insight into the contradictory roles of SATB2 in cancer development.
Keywords: Carcinogenesis, oncogene, special AT-rich sequence-binding protein, tumor suppressor
How to cite this article:
Chen QY, Costa M. Oncogenic and tumor suppressive roles of special AT-rich sequence-binding protein. J Carcinog 2018;17:2
How to cite this URL:
Chen QY, Costa M. Oncogenic and tumor suppressive roles of special AT-rich sequence-binding protein. J Carcinog [serial online] 2018 [cited 2021 Oct 13];17:2. Available from: https://carcinogenesis.com/text.asp?2018/17/1/2/229418
Introduction
As part of the Human Unidentified Gene-Encoded protein database project, special AT-rich sequence-binding protein 2 (SATB2) wasfirst isolated from the human fetal brain cDNA library and later found to reside in 2q32-q33, a gene poor region composed of 733 amino acids weighing approximately 82.5 kDa.[1],[2],[3] The functional domains of SATB2 consist of two CUT domains that aid in the binding to the matrix attachment region (MAR) and a homeodomain at the C-terminus, both of which are highly conserved through evolution and across vertebrate taxa, such as mouse, chicken, and zebrafish.[2],[4],[5]
As a transcription factor, SATB2 plays pivotal roles in various biological developments including osteoblast differentiation, palate formation, craniofacial development, and differentiation of neurons and stem cells.[2], 3, [6],[7],[8],[9],[10],[11] In addition to cell differentiation, SATB2 has also been found to regulate cell stemness and autophagy.[12],[13],[14],[15] While SATB2 is typically expressed in adult tissues, it is also highly expressed in mouse osteoprogenitor cells, indicating an important role in bone growth.[3],[6],[16],[17] An extensive study employing immunohistochemistry-based screening of 65 types of normal human cells revealed that while positive SATB2 expression is found in the neuronal cells of the hippocampus and cerebral cortex, as well as glandular cells of the lower gastrointestinal tract, expression levels are much more conserved in lymphoid, seminiferous duct, and epididymis cells.[18] On the other hand, the expression of SATB2 is notably absent in most other cell types including epithelial cells of the lung, breast, esophagus, small intestine, stomach, thyroid, smooth muscle and skeletal cells, as well as hepatocytes, urothelial, bile duct, renal tubular, and placental cells.[3],[18],[19] The expression difference in various tissues as well as SATB2 expression is seemingly cell-type dependent and may be involved in exerting cell-specific functions.
SATB2 has also been found to act as a MAR-binding protein. Nuclear MARs are AT-rich DNA sequences that nonspecifically bind to the minor groove of the target protein DNA based on structure recognition. This characteristic allows MARs to have many binding sites and on multiple genes.[20],[21],[22],[23],[24] Not only can MARs regulate gene expression, but they are also broadly involved with higher-order chromatin architecture and long-range enhancer functions.[24],[25],[26],[27],[28],[29],[30],[31] As an embryonic MAR-binding protein, the ability to bind to AT-rich DNA elements enables SATB2 to interact with important histone acetylases, deacetylases, and specific metastasis-associated proteins to propagate epigenetic signals and overall chromatin remodeling.[17],[32]
The many important roles of SATB2 in tissue development, differentiation, and overall chromatin remodeling have led researchers to explore its potential functions in cancer development. Existing evidence suggests that SATB2 has varying expression levels in different tumor tissues, and this review provides a comprehensive review of currently available research on the role of SATB2 in different cancers to provide some insight into the contradictory roles of SATB2 in cancer development. Literature search for this review consisted of a basic search on PubMed using keywords “SATB2” and “special AT-rich sequence-binding protein 2.” Each article was then selected for further examination based on the relevance of the title. In consideration to possible missing data from a primary PubMed search, after coding for relevant information, articles listed in the references section were filtered for further review.
Carcinogenesis
Malignant cell transformation is the process of acquiring cancer cell properties, including changes in cell morphology, gene expression, lack of contact inhibition, uncontrolled growth, gain of anchorage-independent growth, and tissue-invasion abilities.[33],[34],[35] The two major classes of genes – oncogenes and tumor suppressor genes – regulate and encode various genes and proteins important for cellular growth and proliferation and thus are directly responsible for regulating cancer development. The balance of control over cell division is compromised when genetic and epigenetic alterations disrupt their functions. However, the identification of oncogenes and tumor suppressor genes is not always straightforward. For example, prominent genes such as Sirtuin 1 and Wilms tumor 1 have been found to act as both tumor promoters and suppressors.[36],[37] Similarly, SATB2 has been found to play opposing roles in different tissues. In fact, SATB2 expression is highly expressed in some tumors, but not others. By compiling nine study cohorts, Magnusson et al., 2011[19] showed that SATB2 is most commonly expressed in colonic carcinomas, including primary (86%) and metastatic adenocarcinomas (81%). On the other hand, noncolonic tumors such as breast (4%), ovarian (3%), lung (6%), and sinonasal (57%) carcinomas, all demonstrated weak SATB2 expression. Nonetheless, the dual functions of SATB2 may obscure accurate cancer therapeutic efforts; thus, it is important tofirst examine how SATB2 functions under different conditions. This article provides a comprehensive review of currently available studies on the role of SATB2 in various cancer tissues and cell lines. Below, the referenced studies are separated into two major sections based on the proposed function of SATB2: tumor promoter and tumor suppressor.
Tumor suppressor
Tumor suppressors are critical for restraining cells from uncontrolled proliferation. SATB2 has been identified as a potential tumor suppressor in several human cancers including colorectal, gastric, and lung [Table 1].[5],[38],[39],[40],[41],[42],[43],[44],[45],[46],[47] Distant metastasis is one of the leading causes of death in non-small cell lung cancer (NSCLC) patients, and epithelial–mesenchymal transition (EMT) has been shown to play an important role in tumor formation, metastasis, and drug resistance.[48],[49] Since SATB2 has also been shown to stimulate EMT in colorectal cancer (CRC), the research team led by Kucuksayan et al., 2016[44] sought to analyze the possible link between SATB2 and transforming growth factor-beta (TGF-β), a prominent external factor important for lung cancer invasion and EMT.[50],[51],[52] In addition to reduced SATB2 expression in NSCLC cells, the researchers also found that inhibition of SATB2 led to increased invasion abilities in H1650 cells and promoted TGF-β-induced EMT in A549 cells. Furthermore, small-interfering RNA (siRNA) knockdown of SATB2 in H1650 cells resulted in mesenchymal morphology as well as loss and upregulation of E-cadherin and N-cadherin, respectively. Overall, knockdown of SATB2 led to greater invasion capacity and thus may be a negative regulator of EMT in NSCLC cells.
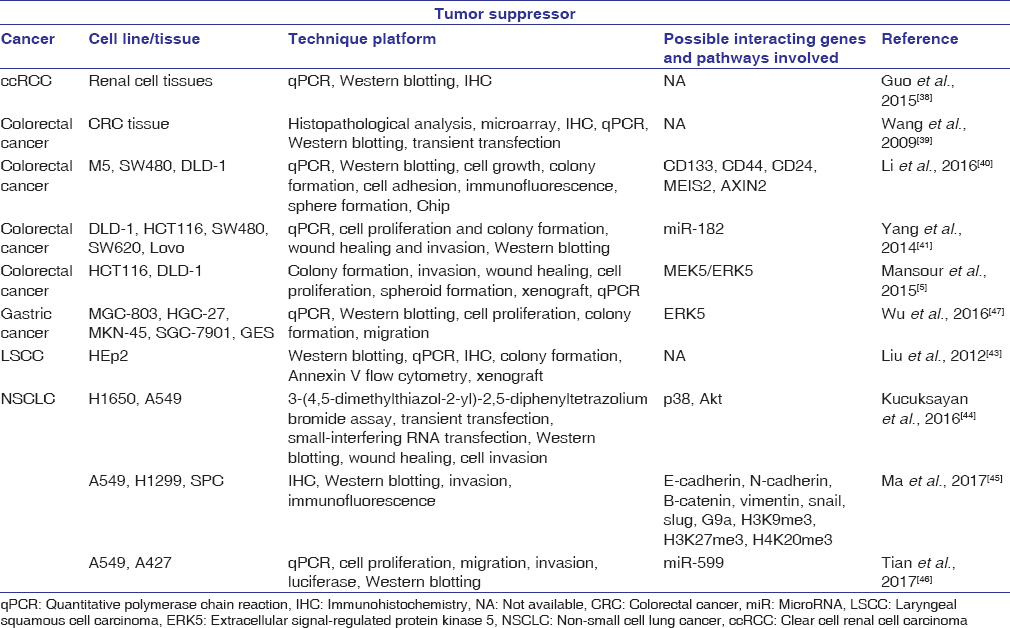 |
Table 1: Studies demonstrating special AT-rich sequence-binding protein 2 as a tumor suppressor gene Click here to view |
SATB2 expression has also been suggested to have favorable effects in CRC.[39] An immunohistochemical analysis using 146 colorectal tumor tissues revealed a correlation between poor disease prognosis and reduced SATB2 expression. In particular, the study suggested that SATB2 expression specifically targeted tumor invasion and cell metastasis. Furthermore, a study led by Li et al., 2016[40] demonstrated that SATB2 expression inhibits transformation properties in human colorectal adenocarcinoma cell lines: SW480 and DLD-1 cells. Specifically, overexpression of SATB2 in SW480 and DLD-1 cells using lentivirus transduction demonstrated reduced proliferation, migration, and colony formation. On the other hand, knockdown of SATB2 in the same metastatic and nonmetastatic CRC cells demonstrated elevated migration and colony-formation abilities. As this study demonstrates, the presence of SATB2 in CRC cells was in part responsible for the reduction in cell transformation properties, including cell proliferation, migration, and colony formation.
A separate study on gastric cancer reported on the correlation between shortened survival rate and the downregulation of SATB2.[47] In gastric cancer tissues and cell lines, both mRNA and protein levels of SATB2 were shown to be downregulated in comparison to normal gastric mucosa tissues and cell lines. The researchers looked further into the effect of extracellular signal-regulated kinase 5 (ERK5), a protein often found to be unregulated in various tumors, and investigated its possible correlation with SATB2 in gastric cancer.[53],[54],[55],[56] The study results suggest that ectopic expression of ERK5 stimulated gastric cancer cell proliferation and migration. Moreover, overexpression of SATB2 was shown to repress ERK5 expression. Collectively, these data are in agreement with the recent studies, which suggest that SATB2 may act as a tumor suppressor.[24],[57],[58]
In laryngeal squamous cell carcinoma (LSCC) tissues, decreased protein and mRNA SATB2 expression has been correlated with higher tumor recurrence and histological grade.[43] Specifically, overall patient survival rate is found to be greater with higher SATB2 expression. Similar results were obtained throughin vitro andin vivo experiments. In LSCC cells (HEp2), overexpression of SATB2 led to decreased cell proliferation and malignant transformation properties. On the other hand, shRNA-induced SATB2 inhibition resulted in greater cell proliferation and malignant-transformation abilities. Furthermore, HEp2 cells overexpressing SATB2 inhibited tumor formation in nude mice. Consistent with a recent study that suggested a correlation between low SATB2 expression and increased tumor invasion and metastasis, this study proposed that low SATB2 expression might be a possible prognostic and risk factor for LSCC.
Clear cell renal cell carcinoma (ccRCC) is the most common type of RCC and is remarkably resistant to chemo- and radio-therapy.[38],[59] As a potential tumor suppressor for colon and LSCC, SATB2 was investigated for its mechanistic effect in ccRCC development.[38] Compared to normal tissues, ccRCC tissues demonstrated a significant reduction in SATB2 expression. In addition, high SATB2 expression was positively correlated with greater overall survival rate for ccRCC patients. These results suggest that SATB2 may act as a tumor suppressor in RCC.
Tumor promoter
In contrast to the above studies, the current research also suggests that SATB2 may act as a tumor promoter. This section serves to examine currently available literature in support of this hypothesis [Table 2]. Evidence shows that SATB2 is overexpressed in several tumors including colorectal carcinoma, Merkel cell carcinoma, and hepatocellular carcinoma.[19],[61],[62],[63],[64] In addition, mRNA levels of SATB2 have also been linked to breast cancer (BCa) grade and rate of survival.[24],[61] More prominent are the findings that SATB2 overexpression can be detected in approximately 85% of CRC tumors and may act as a potential diagnostic marker for colon cancer.[19],[61] The positive correlation between SATB2 expression and tumor development lends perspective to the function of SATB2 as a possible tumor promoter.
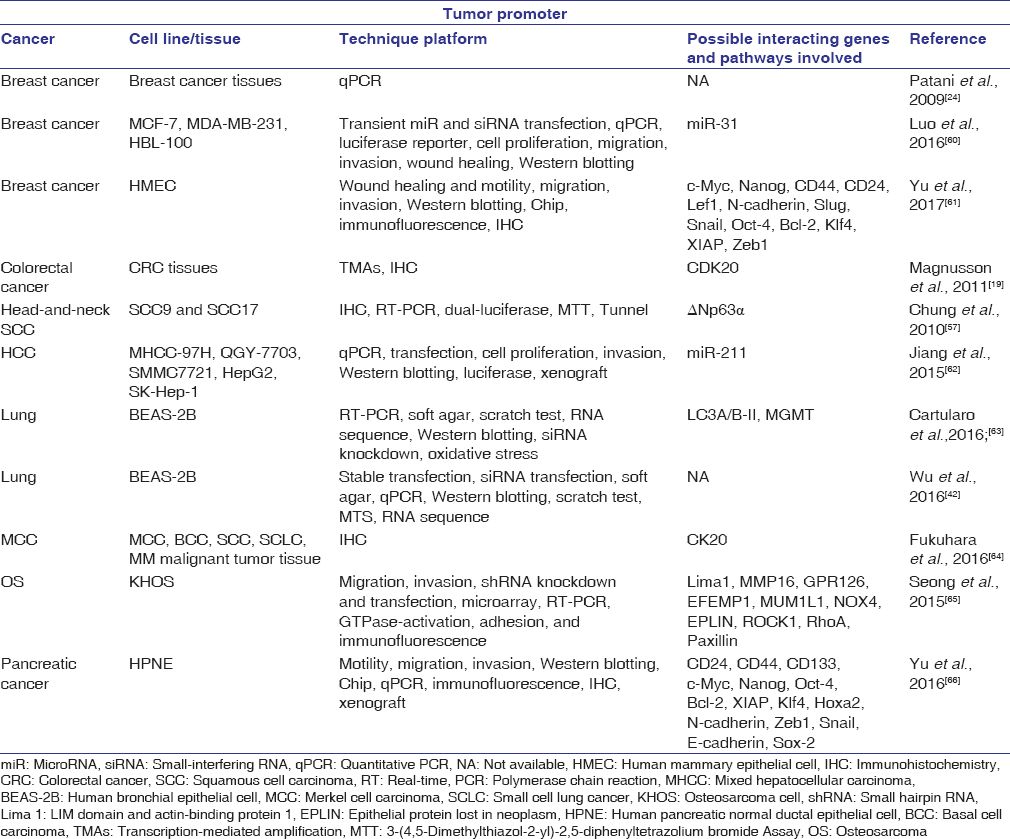 |
Table 2: Studies demonstrating special AT-rich sequence-binding protein 2 as an oncogene Click here to view |
Under normal circumstances, SATB2 is weakly expressed in the lung.[65] However, non-SATB2-expressing cells such as human bronchial epithelial cells (BEAS-2B) displayed elevated protein and mRNA levels of SATB2 on heavy metal-induced transformation.[42],[63],[67] To further explore the effect of SATB2 in heavy metal-transformed cells, a study led by Wu et al., 2016[42] inhibited SATB2 expression in nickel-transformed BEAS-2B cells using shRNA and found that decreased SATB2 expression significantly reduced anchorage-independent growth and cell proliferation. This study demonstrated that SATB2 may in part be responsible for nickel-induced BEAS-2B cell transformation, and similar results may be true for other carcinogenic metals.
Although SATB2 is not detectable in human pancreatic normal ductal epithelial (HPNE) cells, it is highly expressed in pancreatic cancer tissues.[66] To elucidate the molecular mechanism behind HPNE cell transformation, a study led by Yu et al., 2016[66] generated SATB2-expressing HPNE cells using lentiviral-mediated infection. The results indicated that overexpression of SATB2 in HPNE cells led to increased cell stemness, cellular transformation, and tumor formation in nude mice. Furthermore, SATB2 overexpression in HPNE cells was found to induce cell migration and invasion through upregulation of Zeb1 and N-cadherin and inhibition of E-cadherin expression, all important transcription factors for EMT. On the other hand, inhibition of SATB2 in pancreatic cancer cells and cancer stem cells (CSCs) significantly reduced cell proliferation, colony formation, and EMT properties. These data suggest that overexpression of SATB2 can lead to increased cellular proliferation, survival, and pluripotency.
Like BEAS-2B and HPNE cells, SATB2 is undetectable in normal breast tissues and human mammary epithelial cells. However, SATB2 is highly expressed in human BCa cells, primary mammary tissues, and CSCs.[61] Evidence also suggests that SATB2 is highly expressed in BCa tissues and in addition significantly correlated with BCa grade and poor patient survival.[24],[60] To understand the role of SATB2 in BCa development, Luo et al., 2016[60] first examined the expression of miRNA31, a possible tumor suppressor dysregulated in multiple cancers, in BCa tissues and cell lines.[68],[69],[70],[71] Compared with nontumor tissues, BCa tissues displayed significantly reduced miRNA expression. The downregulation of miR-31 was also promoted cell migration and invasion in BCa cell lines. In relation to SATB2, the study suggested that miR-31 is inversely correlated with and acts as a direct target of SATB2. SATB2 has been found to be upregulated in BCa cells, while silencing of the gene led to a reduction in cell proliferation, invasion, and migration. Overall, this study suggests that SATB2 may act as an oncogene in BCa through upstream miR-31 downregulation.
Osteosarcoma (OS) is one of the most common forms of malignant bone tumor. Using immunostaining, Seong et al., 2015[65] discovered that the nuclear expression of SATB2 was present in 93% of primary OS tissues and overexpressed in cells derived from OS metastasis, suggesting an important role in tumor metastasis. SATB2-knockdown OS cells (KHOS) displayed reduced cell migration and invasion, with no significant changes in cell proliferation and viability. Microarray analyses also showed significant differences in the expression levels of 425 genes in control and SATB2-knockdown KHOS cells. Gene Set Enrichment Analysis indicated that a significant number of the 425 genes were involved in cell migration, cytoskeleton organization, and small GTPase signaling pathways. Interestingly, LIM domain and actin-binding protein 1 (Lima1) was found to be substantially upregulated in SATB2-knockdown KHOS cells. Lima1 is known to encode epithelial protein lost in neoplasm (EPLIN), a cytoskeleton-associated protein important for deterring EMT through negatively regulating growth and invasion. High expression of EPLIN in SATB2-knockdown KHOS cells detected by real-time polymerase chain reaction, immunofluorescence assays, and immunoblots further suggests that EMT inhibition is stimulated in the absence of SATB2 expression. In other words, SATB2 may be partially responsible for OS metastasis through indirect regulation of Lima1 and EPLIN proteins.
SATB2 has also been shown to resensitize head-and-neck squamous cell carcinomas (HNSCCs) to chemotherapy and γ-irradiation-induced apoptosis by encouraging dominant-negative (Δ)p63α engagement with p53-family responsive elements.[57] As a pro-survival protein, Δp63α is overexpressed in 80% of HNSCCs and is found to inhibit pro-apoptotic Tap73 β gene and its transcription of subsequent p53-target genes including noxa and puma.[72] The inhibition of p53-target genes is thought to be responsible for promoting HNSCC resistance to chemotherapy-induced cell death.[73] To test the chemo-resistant effect of SATB2 in squamous cell carcinomas, SCC9 and SCC17 cells were transfected with lentivirus to knockdown SATB2 and treated with chemotherapeutic agent – cisplatin. Compared with the control group, SCC9 and SCC17 SATB2-knockdown cells were shown to be more susceptible to cisplatin-induced cell death.[57] The researchers concluded that SATB2 may be an important factor in enhancing cell survival under chemotherapeutic and radiation treatments. Because SATB2 is highly expressed in advanced HNSCC tumors, this finding may help explain the resistant to treatment during late-stage HNSCC.
Conclusion
Since its discovery, many studies have demonstrated the importance of SATB2 in tissue development, cell differentiation, and chromatin structure. In consideration to its wide-ranging functions, recent studies aimed to understand the possible role of SATB2 in cancer development. Specifically, using various tumor tissues and cell lines, the researchers sought to determine the tumor suppressive and oncogenic roles of SATB2; however, the current studies seem to provide contrasting arguments. Through a comprehensive literature search, this review consolidated existing research and grouped the studies into two major categories based on proposed functions of SATB2: tumor promoter and tumor suppressor. Note, some studies included here merely indicate the up- or down-regulation of SATB2 expression in specific tumor tissues rather than analyzing its functional roles in cancer development. As indicated in [Table 1], studies have shown that SATB2 may act as a tumor suppressor in renal, colorectal, and gastric cancers, NSCLC, and LSCCs. On the other hand, studies in [Table 2] suggest that SATB2 may act as a tumor promoter in breast, colorectal, pancreatic, and lung cancers, as well as OS, hepatocellular and Merkel cell carcinomas, and HNSCCs. Interestingly, SATB2 seems to have overlapping functions in colorectal and lung cancers. This may be due to the tested sample of choice in each study. For example, studies conducted by Li et al., 2016[40] and Mansour et al., 2015[5] incorporated both colorectal tumor tissues and cell lines. On the other hand, Magnusson et al., 2011[19] only demonstrated the upregulation of SATB2 in tumor tissues. In a similar sense, different cell lines were used in the studies involving malignant transformation of lung cells and tissues. Furthermore, SATB2 can exert its functions through different pathways. For example, in triple-negative BCa, SATB2 is directly targeted by miR31 to inhibit cell migration and expression,[60] while in osteoblast, differentiation in mouse tissues is regulated by miR-34.[14] Another study reported that SATB2 is capable of directly binding to genes such as Bcl-2, Bsp, c-Myc, XIAP, Klf4, Hoxa2, and Nanog, which are all important for pluripotency and cell survival.[66] These findings show that SATB2 can regulate multiple downstream genes and be targeted by multiple factors. Most importantly, the dual functions of SATB2 in different tissues and cell lines may be dependent on the abundance as well as the spatial and temporal distribution of its downstream targets and upstream regulators. The controversy over why SATB2 serves as a tumor suppressor or tumor promoter in some cancers but not others has not been completely solved, and more discussion will likely continue. Future research should address the mechanistic pathways involved in SATB2-related cancer formation. In addition, more information is needed regarding whether SATB2 activation and suppression are part of an early or late event in different cancers. This review provides a comprehensive list of existing studies and serves as a reference for comparing the opposing roles of SATB2 in different cancers and cell lines. Understanding the mechanistic role of tumor genes is essential for targeted cancer therapy; therefore, further research is needed to analyze the interacting proteins and pathways responsible for the contrasting responses of SATB2 in various types of cancers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. | |
| 25. | |
| 26. | |
| 27. | |
| 28. | |
| 29. | |
| 30. | |
| 31. | |
| 32. | |
| 33. | |
| 34. | |
| 35. | |
| 36. | |
| 37. | |
| 38. | |
| 39. | |
| 40. | |
| 41. | |
| 42. | |
| 43. | |
| 44. | |
| 45. |
Ma YN, Zhang HY, Fei LR, Zhang MY, Wang CC, Luo Y, et al. SATB2 suppresses non-small cell lung cancer invasiveness by G9a. Clin Exp Med 2017. DOI:https://doi.org/10.1007/s10238-017-0464-3.
 |
| 46. | |
| 47. | |
| 48. | |
| 49. | |
| 50. | |
| 51. | |
| 52. | |
| 53. | |
| 54. | |
| 55. | |
| 56. | |
| 57. | |
| 58. | |
| 59. | |
| 60. | |
| 61. | |
| 62. | |
| 63. | |
| 64. | |
| 65. | |
| 66. | |
| 67. | |
| 68. | |
| 69. | |
| 70. | |
| 71. | |
| 72. | |
| 73. |
