Phu N Tran1, Thomas H Taylor2, Samuel J Klempner3, Jason A Zell4
1Department of Medicine, Division of Hematology/Oncology, University of California, Irvine, CA, USA
2Department of Epidemiology and Genetic Epidemiology Research Institute, University of California, Irvine, CA, USA
3The Angeles Clinic and Research Institute; Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA
4Department of Medicine, Division of Hematology/Oncology; Department of Epidemiology and Genetic Epidemiology Research Institute, University of California, Irvine, CA, USA
| Date of Submission | 08-Mar-2017 |
| Date of Acceptance | 12-Jul-2017 |
| Date of Web Publication | 18-Sep-2017 |
Correspondence Address:
Jason A Zell
Department of Medicine, Division of Hematology and Oncology, University of California, Irvine, 101 The City Drive South, Building 56. Orange, CA 92868
USA
Source of Support: None, Conflict of Interest: None
Abstract
Background: African Americans and Hispanics are reported to have higher mortality from esophageal cancer (EC) than Caucasians. In this study, we analyzed the independent effects of race, gender, treatment, and socioeconomic status (SES) on overall survival (OS). Methods: Data for all EC cases between 2004 and 2010 with follow-up through 2012 were obtained from the California Cancer Registry. We conducted descriptive analyses of clinical variables and survival analyses by Kaplan–Meier and Cox proportional hazards methods. Results: African Americans and Hispanics were more likely to be in the lower SES strata and less likely to receive surgery than Caucasians in this cohort. The proportion of patients receiving chemotherapy and radiotherapy was similar across different racial/ethnic groups. After adjustment for stage, grade, histology, treatments, and SES in multivariate analyses, the mortality risk in African Americans (hazard ratio [HR] 0.96, 95% confidence interval [CI] 0.85–1.07) and Hispanics (HR 0.96, 95% CI 0.89–1.07) did not differ from Caucasians (HR = 1.00, referent), with histology, SES, and surgery largely accounting for unadjusted OS differences. We also observed that African American men had higher adjusted risk of death relative to Caucasian men (HR 1.24, 95% CI 1.07–1.42), but this effect was not observed for African American women compared to Caucasian women (HR 1.12, 95% CI 0.94–1.35). Conclusions: Race is not an independent risk factor for OS in our population-based analysis of EC cases. Rather, observed differences in OS by race/ethnicity result from differences in cancer histology, SES, surgery, and gender. Our findings support further health disparities research for this disease.
Keywords: Esophageal cancer, ethnicity, outcomes, race, survival disparity
| How to cite this article: Tran PN, Taylor TH, Klempner SJ, Zell JA. The impact of gender, race, socioeconomic status, and treatment on outcomes in esophageal cancer: A population-based analysis. J Carcinog 2017;16:3 |
| How to cite this URL: Tran PN, Taylor TH, Klempner SJ, Zell JA. The impact of gender, race, socioeconomic status, and treatment on outcomes in esophageal cancer: A population-based analysis. J Carcinog [serial online] 2017 [cited 2021 Oct 13];16:3. Available from: https://carcinogenesis.com/text.asp?2017/16/1/3/215051 |
Introduction
Esophageal cancer (EC) is a deadly disease with major health implications worldwide. It is the eighth most common cancer but the sixth most common cause of cancer-related death.[1] The incidence of EC has been rising in the US over the past 20 years, with 16,910 new diagnoses and 15,690 deaths predicted in 2016.[2] Since the 1970s, the incidence of adenocarcinoma has risen from 10% of all US EC cases to more than 60%.[3] The increased proportion of esophageal adenocarcinoma (EAC) has been particularly dramatic among Caucasian men, with a concomitant decrease in the incidence of squamous cell carcinoma (SCC), particularly among African American men.[4] Meanwhile, the incidence of esophageal carcinoma has remained stable among Asian Americans, with EAC at 0.7 cases per 100,000 and SCC at 3.9/100,000.[5] Esophageal SCC (ESCC) has been reported to carry higher mortality risk than adenocarcinoma.[6],[7]
Overall survival (OS) after diagnosis of EC remains poor, and multiple studies have suggested worse outcomes in African Americans versus Caucasians.[8],[9],[10],[11] While the higher prevalence of SCC subtype in African Americans may partly explain survival disparities relative to Caucasians, their overall lower socioeconomic status (SES) and poorer access to care may play a larger role. In a prior study, African Americans were more likely to present at advanced stage and younger age but were less likely than Caucasians to have surgery.[9] Few studies address survival disparities for Hispanics with EC. One study reported lower surgery rates and higher unadjusted mortality among Hispanic patients compared to Caucasian patients with EC.[11]
Given racial diversity in the US, identifying factors associated with differences in long-term survival of EC across different racial/ethnic subgroups may have important clinical implications. In this study, we performed a population-based analysis of California Cancer Registry (CCR) data to determine if the previously reported associations of race/ethnicity with survival after EC diagnosis are independent of gender, histology, SES, and treatments such as chemotherapy, radiation, and surgery.
Methods
Patient population
Population-based data were obtained from primary EC cases diagnosed in the state of California and reported to the CCR. Demographic variables (age, gender, race/ethnicity, and SES) and clinical characteristics (stage, tumor grade, histology, treatment modality within 6 months of diagnosis, and survival) were extracted from the CCR database. Cases diagnosed at autopsy or recorded solely from death certificate information were excluded. Race/ethnicity in the CCR data is grouped as non-Hispanic White (Caucasian), non-Hispanic Black (African American), Hispanic, and non-Hispanic Asian/Pacific Islanders (Asian). Because of small numbers, we excluded Native Americans and those of unknown race/ethnicity, comprised 1.5% of the study population. Cases diagnosed between 2004 and 2010 were included in the cohort, with follow-up through December 31, 2012 (the cutoff point of our most recent CCR dataset, to allow at least 2 years follow-up period for all cases). We chose to analyze data from 2004 onward as this period reflects contemporary EC treatments and since this period commences with the introduction of the CCR cumulative stage variable, which accounts for American Joint Committee on Cancer (AJCC) stage at diagnosis. Of 6976 patients in the study, the proportion of individuals with missing surgery, chemotherapy, and radiotherapy was 5.21%, 0.58%, and 0.81%, respectively. These individuals were excluded from survival analyses.
Cases of EC were identified as SEER site recode 21010 and ICD-O-3 C150-C159. Histology codes include adenocarcinoma (8140–8151, 8154–8231, 8243–8245, 8250–8576) and SCC (8000–8131, 8980–8981). Tumor staging was defined by the AJCC Tumor, Node, and Metastasis 7th Edition staging classification.[12] Tumor grade is classified in the CCR database as well differentiated, moderately well differentiated, poorly differentiated, undifferentiated/anaplastic, and unknown. SES data in the CCR are coded by a derived variable accounting for summary measures of the census tract in which the patient resides at diagnosis (e.g., education, income, cost of living, and occupation type). SES was categorized from lowest to highest quintile. This composite SES variable has been used in multiple epidemiologic publications on various types of cancers.[13],[14],[15],[16] In terms of treatments, the variable surgery is defined as the most extensive type of surgery performed during thefirst course of treatment for the tumor and within 6 months of diagnosis. Similarly, radiation and chemotherapy indicate that patients received these treatments within 6 months of diagnosis.
Statistical analysis
Analyses of categorical variables were done by Fisher’s exact test. Comparison of continuous variables between or among groups was done using the Kruskal–Wallis test. Survival time was recorded in years from the date of diagnosis to the date of death from any cause (OS) or loss to follow-up. The Kaplan–Meier method was used to estimate the OS curve for race/ethnicity, each SES category, and histology. Differences in mortality between/among race and SES groups were analyzed using the log-rank test. Cox proportional hazards regression model was used to estimate independent relationships among race, ethnicity, and mortality. By default, age at diagnosis and gender were incorporated in this model. The model was sequentially adjusted for patient factors, tumor factors, and receipt of surgery, chemotherapy, and radiotherapy. Diagnostics indicated that the proportionality assumption was reasonable in these data. All tests of statistical significance were two-sided, and statistical significance was defined as P ≤ 0.05. All statistical analyses were computed using SAS © 9.3 software (Cary, NC, USA).
Results
Demographic characteristics
A total of 6976 patients with primary EC were identified from the CCR between 2004 and 2010 with follow-up through December 31, 2012 [Table 1]. Caucasians comprised the majority of the study cohort (71%), followed by Hispanics (13.8%), Asians (8.2%), African Americans (5.6%), and Native Americans/other groups (1.5%). EC occurred more commonly in men and among cases within age 60–69 and 70–85 years [Table 1]. Consistent with national trends, adenocarcinoma was more common than SCC histology (58.3 vs. 41.7%) in our study cohort. SCC predominated in African Americans (79.1%) and Asians (75.4%), while adenocarcinoma was more commonly in Caucasians (65.6%) and Hispanics (56%). In the analysis of stage at presentation, the largest proportion of patients presented with Stage IV disease (32.7%). Stage I, II, and III constituted 15.9%, 13.8%, and 17.0%, respectively. Nearly 19% of patients were classified as unknown stage. African Americans and Caucasians had a similar distribution in EC stage at diagnosis, while Hispanics were more likely to present at advanced stages. The majority of African Americans and Hispanics were in the lower SES strata, while a higher proportion Caucasians and Asians were in the higher SES. The proportion of female EC cases was greater among African Americans than Caucasians (40.2 vs. 23.2%; P < 0.0001) [Table 1].
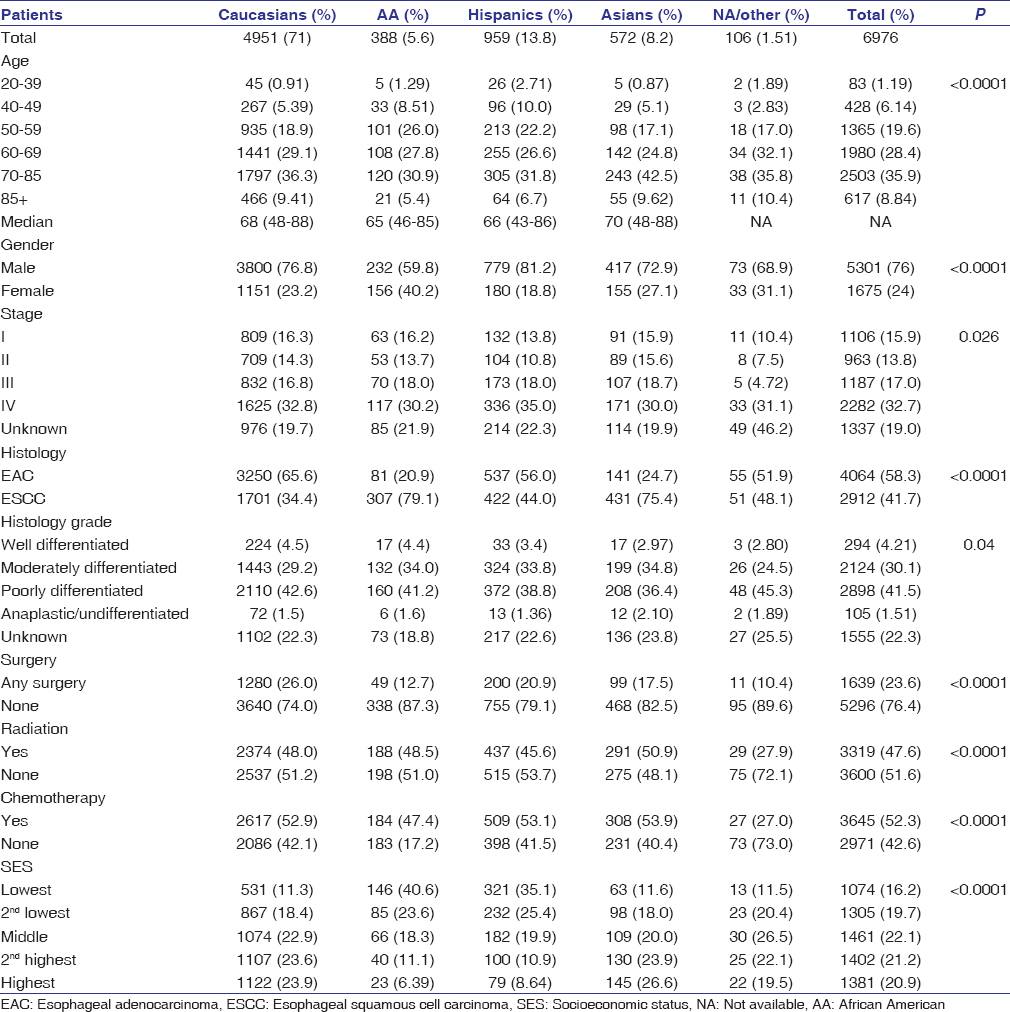 |
Table 1: Clinical and demographic data for patients with esophageal cancer in the California Cancer Registry, 2004-2010 with follow-up through December 31, 2012 Click here to view |
Treatment by stage and race
African Americans were less likely than Caucasians to undergo surgery in Stages I–III disease (23.7% vs. 45.1%; P < 0.0001) [Table 2]. Asians and Hispanics also had a lower frequency of surgery than Caucasians, but the differences were less striking. The frequency of surgery was correlated with socioeconomic factor (SES) and histology in our study. For instance, individuals with Stage I EC who resided in the highest SES strata were more likely to have surgery than those in lowest SES strata (44.3% vs. 25.9%). Among patients with Stage I–III EAC, the proportion of Caucasian, African American, Hispanic, and Asian patients receiving surgery was 53.4%, 54.5%, 40.9%, and 52.5%, respectively. On the other hand, the proportion of Caucasian, African American, Hispanic, and Asian patients with Stage I–III ESCC receiving surgery was 27.8%, 16.4%, 28.1%, and 22.3%, respectively. While African Americans and Caucasians were overall equally likely to receive chemotherapy (47.4% vs. 52.9% combined in all stages; P = 0.502), African Americans received less chemotherapy in Stages III and IV compared to Caucasians [Table 2]. Treatment with radiation therapy was similar across all racial groups in combined stages. The vast majority of patients in the unknown stage group did not receive surgery [Table 2].
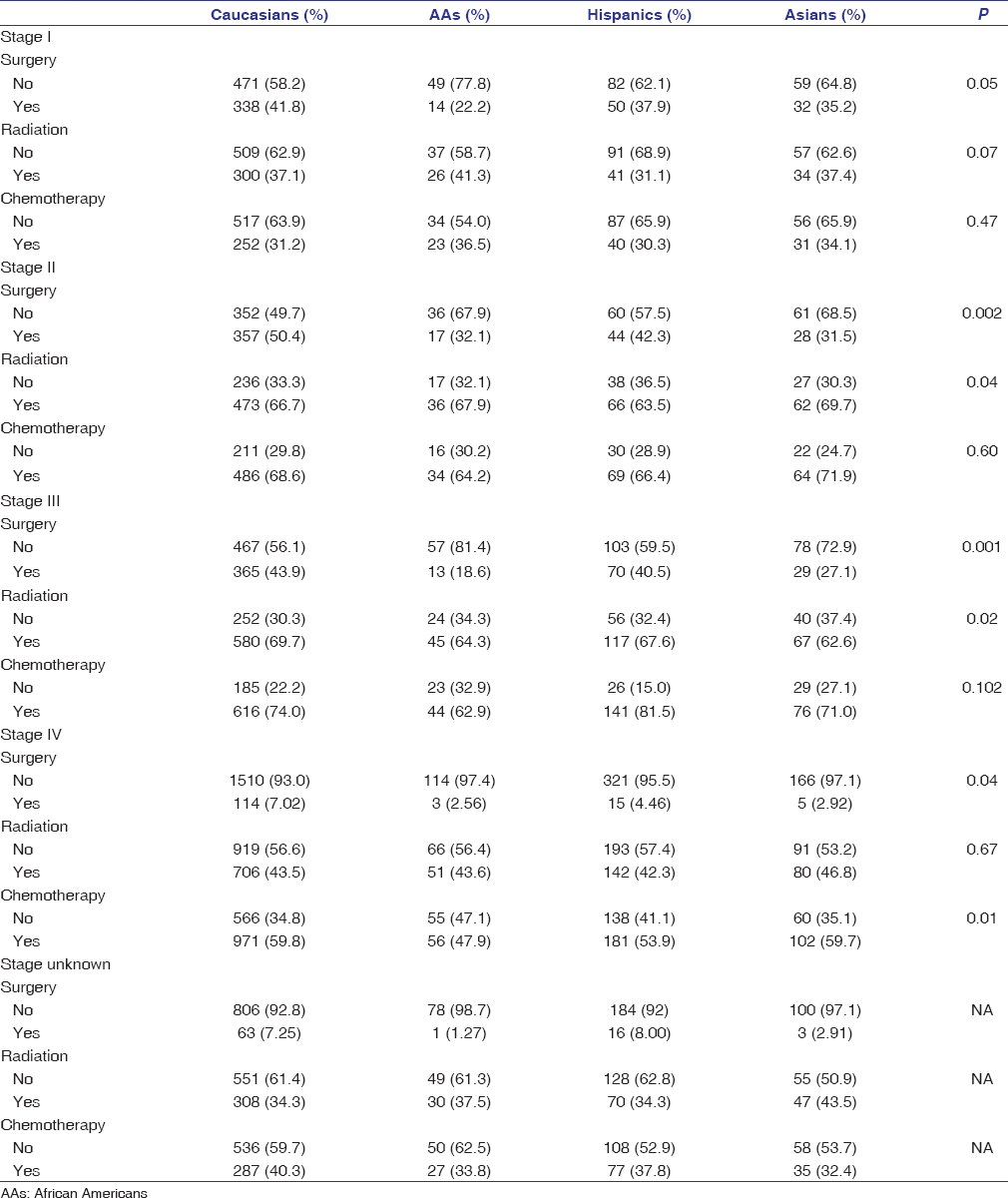 |
Table 2: Proportion of esophageal carcinoma patients treated with surgery, radiation, and chemotherapy, stratified by stage among Caucasians, African Americans, Hispanics and Asians; California Cancer Registry, 2004-2010 with follow-up through December 31, 2012 Click here to view |
Survival analysis by race/ethnicity
At the time of data extract for analysis, only 933 out of 6976 patients (13.6%) were alive. The major cause of death was EC itself with 5011 deaths (84.3%). By univariate analysis, the median OS for Caucasians, African Americans, Hispanics, and Asians was 9 (95% confidence interval [CI] 9–10), 8 (95% CI 6–10), 9 (95% CI 8–10), and 9 (95% CI 8–11) months, respectively. The OS rates at 1, 3, and 5 years were 42%, 19%, and 14% for Caucasians; 38%, 18%, and 12% for African Americans; 39%, 18%, and 13% for Hispanics; and 41%, 26%, and 15% for Asians. African Americans had inferior OS versus Caucasians with Stage I–III EC [Figure 1]. In Stage IV, there was no survival difference [Figure 2]. When examining the effect of gender on median OS, African American males and Hispanics females had the lowest median OS of 7 months (95% CI 6–9) and 6 months (95% CI 5–9), respectively. We attempted to analyze whether the differences in treatment and stage at presentation could contribute to gender-specific survival by race/ethnicity, but the sample size for African Americans, Hispanics, and Asians was too small to derive any meaningful conclusions. There was a clear difference in OS across different SES groups, P < 0.0001 [Figure 3]. SCC carried an increased mortality risk relative to adenocarcinoma with hazard ratio (HR) for death of 1.17 (P < 0.0001).
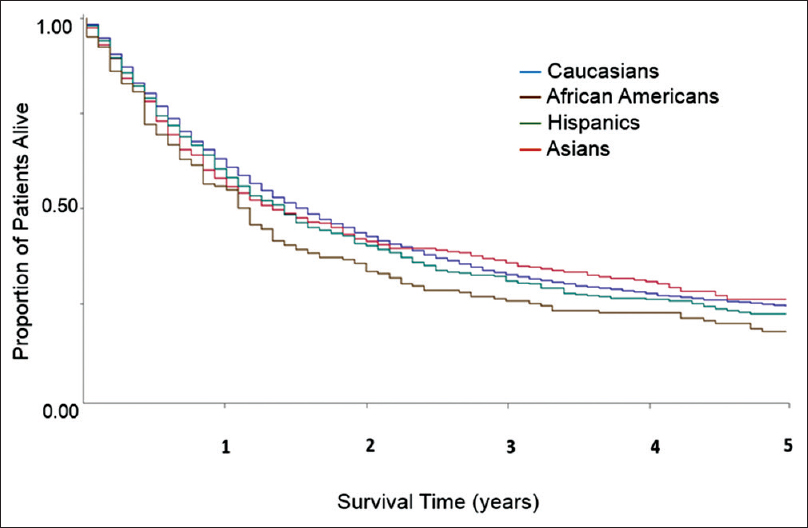 |
Figure 1: Univariate overall survival by race/ethnicity in patients with esophageal cancer Stage I–III. Data from California Cancer Registry 2004–2010 with follow-up through December 31, 2012 Click here to view |
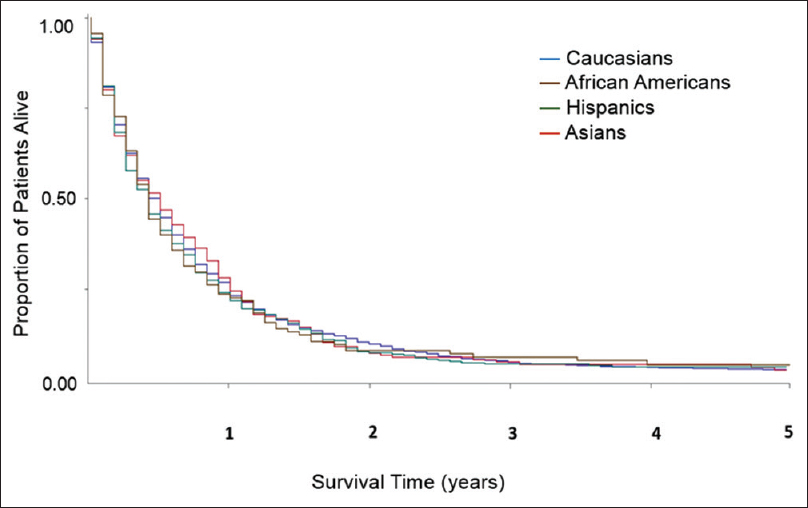 |
Figure 2: Univariate overall survival by race/ethnicity in patients with esophageal cancer Stage IV. Data from California Cancer Registry 2004–2010 with follow-up through December 31, 2012 Click here to view |
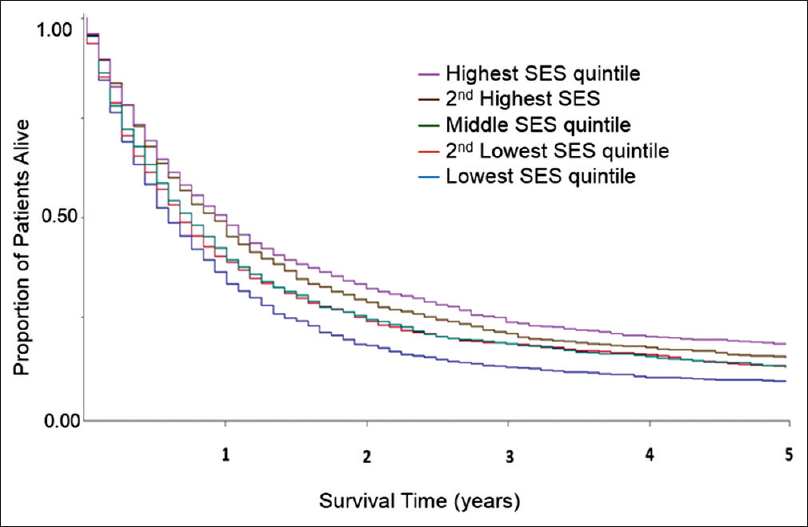 |
Figure 3: Univariate overall survival by socioeconomic status in patients with esophageal cancer in all stages (n = 6976; P < 0.0001). Data from California Cancer Registry 2004–2010 with follow-up through December 31, 2012 Click here to view |
African Americans and Hispanics had increased unadjusted overall mortality risk relative to Caucasians with HR of 1.18 (CI 1.05–1.32, P = 0.004) and 1.12 (CI 1.04–1.21; P = 0.003), respectively. After adjustment for stage, grade, histology, treatments, and SES in multivariate analyses, the mortality risk in African Americans (HR 0.96, 95% CI 0.85–1.07) and Hispanics (HR 0.96, 95% CI 0.89–1.07) did not differ from Caucasians (HR = 1.00, referent). Adjustment for histology, surgery, and SES had the greatest impact on OS estimates for African Americans while adjustment for chemotherapy and radiation therapy had minimal effect [Table 3]. Among Hispanics, adjustment for tumor staging, access to surgery, and SES all significantly impacted OS differences. Separate multivariate analysis of OS based on gender and race showed that African American men had inferior prognosis relative to Caucasian men: HR 1.24 (95% CI 1.07–1.42; P = 0.003). However, observed mortality risk was not statistically different when comparing African American women to Caucasian women: HR 1.12 (95% CI 0.94–1.35; P = 0.236). Similar analysis for Hispanics based on gender and race/ethnicity revealed that among Hispanics, both men and women had increased overall mortality risk compared to Caucasians with HRs of 1.10 among men (1.01–1.20; P = 0.031) and 1.22 among women (1.03–1.45; P = 0.024). Adjustment for stage, surgery, and SES decreased the HRs for Hispanic men and women to approximate that of Caucasians.
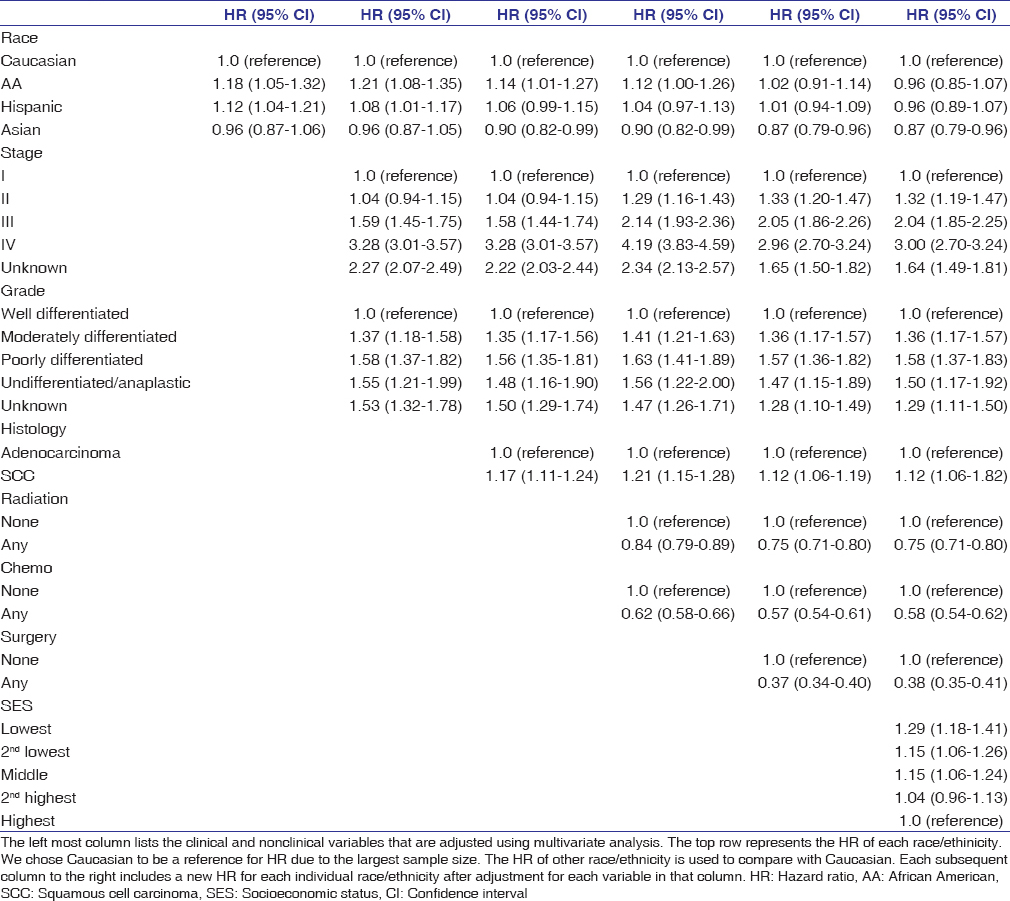 |
Table 3: Multivariate overall mortality analysis of esophageal cancer cases by ethnicity using Cox proportional hazards regression analysis, California Cancer Registry, 2004-2010 with follow-up through December 31, 2012 Click here to view |
Discussion
Epidemiologic studies have suggested inferior EC outcomes in African American and Hispanic populations. However, prior reports have been limited in their inability to account for the impact of SES or treatment with chemotherapy-information that is available in the CCR but not readily available in SEER. Here, we present population level analyses from cancer registry data revealing that after accounting for differences in typical clinicopathologic features along with treatment and SES, race/ethnicity is not an independent risk factor for mortality in EC. To our knowledge, this is the largest study that assesses relative contributions of SES and treatment on EC outcomes and it provides deeper understanding of prognostic factors for EC survival.
Histology is a known prognostic factor in EC, and we observed significant clinical and histologic variation by race in this population-based study. Consistent with published reports, individuals with SCC were observed to have increased overall mortality risk compared to those with adenocarcinomas (HR 1.17; P < 0.0001). Across race/ethnic categories, we observed that African Americans and Asians had higher proportions of ESCC than EAC while Caucasians had more EAC than ESCC. The greater proportion of ECSS in African Americans and Asian may be related to genetics, risk factors such as tobacco, alcohol, diets high in processed/red meat and low in fruits and vegetables, or both genetic and environmental factors.[17],[18],[19] Along this line of inquiry, recent studies have described an association between genetic polymorphism and ECSS. Significant associations were found between the ALDH2 * 1*2, CYP1A1 Val allele, and p53 codon genotypes and increased risk of squamous EC.[20] There is a need for more research on lifestyle modification and genetic polymorphism on EC development and clinical outcomes in various race/ethnic groups. Prior reports have suggested that different lymphatic patterns of spread, higher prevalence of comorbidities, and other primary tumors among patients with esophageal squamous carcinoma likely contribute to inferior survival compared to those with adenocarcinoma.[6],[7] Our multivariate analysis model revealed that adjustment for histology reduces the observed risk of death among African Americans versus Caucasians (i.e. HR from 1.21 to 1.14) [Table 3]. However, histology does not solely explain the survival disparity in African Americans. Despite having a similar proportion of ESCC as Asians, African Americans were less likely to have surgery, predominantly resided in lower SES strata, and had increased overall mortality risk compared to Asians.
The proportion of patients who received surgical intervention is low across all racial groups in our study but consistent with previous reports. Even in Stage I disease, where surgery is potentially curative, only 41.8% of Caucasians and just 22.2% of African Americans underwent surgery in our study. Paulson et al. reported that the overall surgery rate was only 34.1% for Stage I–III disease (36.8% White vs. 19.2% non-White) despite the improved median survival in surgical patients versus nonsurgical patients (620 vs. 381 days).[21] Disparities in surgery among African American patients can be partly attributed to higher proportion of ESCC histology. ESCC tends to be in the upper third of the esophagus and is more likely treated with definitive chemoradiation rather than surgery. Compared to Caucasians, the proportion of African Americans receiving surgery in our study was similar for Stage I–III EAC (53.4% vs. 54.5%) but lower for Stage I–III ESCC (27.8 vs. 16.4%). We also observed that patients who resided in lower SES strata (African Americans > Caucasians in our dataset) received less surgery than those who resided in higher SES strata (25.9 vs. 44.3%). Consequently, the lower rate of surgery among African Americans may be associated with a higher prevalance of ESCC and lower SES status, in this population compared with Caucasians. Prior studies reported that lower access to health care,[22] poor patient–physician interactions,[23] and patient preference [24],[25] may explain low surgery rates in African Americans. African Americans in one prior report were less likely to be evaluated for surgery, and if evaluated, they were less likely to undergo surgery.[26] The higher comorbidity score observed in African Americans was thought to reflect their lower surgical rates.[26]
The importance of curative-intent surgical evaluation is highlighted by our findings that disparity in OS between African Americans and Caucasians was mostly observed in Stage I–III and not Stage IV [Figure 1] and [Figure 2]. Furthermore, in multivariate analyses, adjustment for surgery and SES eliminated the observed overall mortality differences between African Americans and Caucasians. Among the three major treatment types, surgery was strongly associated with mortality risk reduction while chemotherapy and radiation had little impact on survival disparities. In addition, our data suggest that African Americans received chemotherapy and radiotherapy at rates similar to Caucasians, which may explain the smaller impact of these treatment modalities on survival disparities.
In addition to histology, surgery, and SES, gender appears to impact survival outcomes in African Americans. Male gender has been associated with more advanced disease stage and lower survival in EC.[27] In multivariate analysis for OS based on gender, we observed that African American men had increased overall mortality risk compared to Caucasian men 1.24 (95% CI 1.07–1.42; P = 0.003) while African American women had similar OS to Caucasian women 1.12 (95% CI 0.94–1.35; P = 0.204). Thus, the OS disparity in the African American population was mostly driven by African American men.
This historical prospective study shares limitations of other cancer registry-based studies. The CCR does not contain individual level data such as comorbid conditions, patient–physician interaction, income, or specific chemotherapeutic agents received. Such factors vary across race/ethnicity and could affect both access to and participation in the complex multidisciplinary health care required for optimal EC treatment. Advancements in staging technologies may influence tumor staging, and “stage drift” is a known confounder when applying older data to new staging systems. The high proportion of unknown stage (nearly 20% of cases) was likely due to the high proportion of patients who did not have surgery. Patients with unknown stage in this study received similar treatment to those with Stage IV disease, suggesting that they likely had advanced disease at diagnosis.
Conclusions
Our results indicate that race/ethnicity is not an independent risk factor for mortality in EC after adjustment for key clinical and demographic variables. Adjustment for stage, surgery, and SES had the greatest associations with overall mortality risk reduction in EC cases, uncovering mortality disparities between African Americans and Caucasians. The low proportion of cases receiving surgery among Caucasian and non-Caucasian patients with locoregional EC in our study suggests that surgery is underutilized in this disease. In particular, the low proportion of cases receiving surgery among African Americans may be due to the higher proportion of ESCC and lower SES in this population. Collectively, our observations in this population-based study of EC support further health disparity-focused research on this aggressive malignancy.
Financial support and sponsorship
Funding was provided by a Supplemental Research Grant from the Department of Medicine at University of California, Irvine.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. | |
| 25. | |
| 26. | |
| 27. |
| Authors |
Phu N. Tran: Department of Medicine, Division of Hematology and Oncology, University of California, Irvine, CA, USA
Thomas H. Taylor: Department of Epidemiology and Genetic Epidemiology Research Institute, University of California, Irvine, CA, USA
Samuel J. Klempner: The Angeles Clinic and Research Institute, Los Angeles, CA, USA. Samuel Oschin Comprehensive Cancer Center, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Jason A. Zell: Department of Medicine, Division of Hematology and Oncology, University of California, Irvine, CA, USA
| Figures |
[Figure 1], [Figure 2], [Figure 3]
| Tables |

