Shankar Sharan Singh1, Madan Lal Brahma Bhatt1, Vandana Singh Kushwaha1, Anshuman Singh2, Rajendra Kumar1, Rajeev Gupta1, Devendra Parmar2
1Department of Radiotherapy, King George’s Medical University, Lucknow, Uttar Pradesh, India
2Division of Developmental Toxicology, CSIR n Institute of Toxicology Research, Lucknow, Uttar Pradesh, India
| Date of Submission | 09-Jul-2016 |
| Date of Acceptance | 11-Apr-2017 |
| Date of Web Publication | 19-Jun-2017 |
Correspondence Address:
Madan Lal Brahma Bhatt
Department of Radiotherapy, King George’s Medical University, Lucknow – 226 003, Uttar Pradesh
India
Source of Support: None, Conflict of Interest: None
Abstract
BACKGROUND: Matrix Metalloproteinase 13 (MMP13) is a member of collagenase family and it is involved in the degradation of extracellular matrix and basement membrane protein. It is thought to be associated with tumor invasion and metastasis. Elevated MMP13 expression has been found in carcinoma of the breast, urinary bladder, head and neck and others. It is observed that MMP13 gene is also correlated with radiation response in OSCC (Oral squamous cell carcinoma) cell line based study. The present study correlates the MMP13 expressions with clinicopathological parameters and radiation response in OSCC patients. MATERIALS AND METHODS: The MMP13 mRNA levels were determined by employing qRT-PCR (real-time quantitative reverse transcriptase-polymerase chain reaction). RESULTS: We observed high expression of MMP13 mRNA in OSCC patients when compared with matched controls. Statistically significant up regulation of MMP13 mRNA expression was found in tobacco chewers, advanced T-stage (p < 0.001) and lymph node metastasis (p < 0.01). MMP13 mRNA levels were also elevated in non responders as compared to responders to radiation treatment. CONCLUSIONS: To the best of our knowledge, this is the first report that indicates role of MMP13 in radiation response in OSCC patients and could be used as potential bio-marker for radiotherapy treatment in OSCC patients.
Keywords: Matrix Metalloproteinase 13, oral squamous cell carcinoma, radiation response
| How to cite this article: Singh SS, Bhatt ML, Kushwaha VS, Singh A, Kumar R, Gupta R, Parmar D. Role of matrix metalloproteinase 13 gene expression in the evaluation of radiation response in oral squamous cell carcinoma. J Carcinog 2017;16:2 |
| How to cite this URL: Singh SS, Bhatt ML, Kushwaha VS, Singh A, Kumar R, Gupta R, Parmar D. Role of matrix metalloproteinase 13 gene expression in the evaluation of radiation response in oral squamous cell carcinoma. J Carcinog [serial online] 2017 [cited 2021 Oct 13];16:2. Available from: https://carcinogenesis.com/text.asp?2017/16/1/2/208418 |
Introduction
Oral squamous cell carcinoma (OSCC) is the 12th most common cancer occurring worldwide. It is more common in males and accounts for more than 30% of all cancers in India.[1] Radiation therapy with or without chemotherapy is used as a primary or adjuvant treatment in advanced OSCC.[2] Despite advances in treatment, only 50% of the patients who underwent radiotherapy have sustained response.[3] It may be due to variability in tumor genetics and biology. Currently, there is no available marker which can reliably predict the radiotherapy response in such patients.
Matrix Metalloproteinase 13 (MMP13) gene encodes a protein, involved in the breakdown of extracellular matrix in normal physiological processes. Previous studies demonstrated that MMP13 is highly overexpressed in pathological conditions, such as arthritis and human carcinomas.[4] MMP13 is the essential protein for tumor invasion and metastatic spread and its expression may help predict the radiotherapy response and prognosis in OSCC patients. Our objective was to examine the expression of MMP13 in relation to clinicopathological features of OSCC patients and with their radiotherapy response.
Materials and Methods
Between 2010 and 2013, 160 histopathologically confirmed tissue samples from OSCC patients (as recommended for the radiotherapy treatment) as well as eighty matched controls were obtained from King George’s Medical University, Lucknow. The study was approved by the Institutional Ethics Committee, and written informed consent was obtained from all participants before the recruitment in the study. The patients had not received prior radiotherapy, chemotherapy, or neoadjuvant therapies for their cancer or any other disease in the past. The characteristics of the OSCC patients including age, clinical stage (TNM classification defined by the American Joint Committee on Cancer (AJCC) 2010, seventh edition)[5] and habits (Tobacco, alcohol, smoking) were assessed by Radiation Oncologist.
Analysis of mRNA expression by real-time quantitative reverse-transcriptase polymerase chain reaction
The tissue specimens of OSCC patients and controls were collected in TRIzol Reagent (Ambion, Life Technology) and immediately homogenized with a T25 basic homogenizer (IKA Labortechnik, Staufen, Germany). Total RNA was isolated according to TRIzol Reagent protocol and stored at −80°C for further use. The total RNA of each sample was reverse transcribed to cDNA using verso cDNA synthesis kit (Thermo Scientific, EU, Lithuania) according to the manufacturer’s protocol. Real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) analysis was performed according to the manufacturer protocol (Takara SYBR fast qPCR kit, Takara Biosystems). The qRT-PCR reactions were performed using Applied Biosystems 7900HT real-time PCR system, and reaction was carried out in triplicate. Beta-actin was taken as an endogenous control and the cDNA from matched control tissue (RQ =1) was utilized for the normalization of test samples. The primer sets specific for MMP13 (Forward: 5′-TTCCCACAGTGCCTATTGATAC-3′, Reverse: 5′-ATCAACAGTGTCTCTGAGCACAA-3′) and for beta-actin (Forward: 5′-GCACA GAGCCTCGCCTT-3′, Reverse: 5′-GTTGTCGACGACGAGCG-3′) were used. An initial incubation of enzyme denaturation at 95°C for 10 min followed by 45 rounds of amplification at 95°C (10 s) for denaturation, 58°C–65°C (10 s) for annealing, and 72°C (30 s) for extention for performing qRT-PCR. Relative change in mRNA levels between tumor and matched normal control tissue was calculated using 2ïΔΔct method.
Proposed methodology
All patients were subjected to radiotherapy using telecobalt radiotherapy machine. A dose of 70 Gy of radiation was delivered in 7 weeks with 2 Gy fraction size, 5 days a week (46 Gy to primary and whole neck followed by 24 Gy after sparing the cord) with concurrent cisplatin 35 mg/m 2/week. Radiation response was evaluated 1 month after the completion of radiotherapy treatment using WHO criteria.[6]
Statistical analysis
One-way ANNOVA using Turkey’s multiple comparison test was used to compare the mRNA expression levels between different groups in both cases and controls. Comparisons were made between categorical groups by Chi-square test. A two-tailed P < 0.05 was considered statistically significant.
Results
Matrix metalloproteinase 13 mRNA expression and basic characteristics of oral squamous cell carcinoma patients
The demographic and clinicopathological parameters of OSCC patients are summarized in [Table 1]. The median age of patients was 48 years. Among 160 patients, majority were males (76.2%), with majority having lesion at buccal mucosa (55%). Out of 122 males, tobacco chewing habit was present in 80 (65.6%) patients, 68 (55.7%) patients were smokers, and 63 (51.6%) patients had a history of alcohol consumption, while in females, 22 (57.9%) were tobacco chewers, 16 (42.1%) were smokers, and 17 (44.7%) patients had alcohol consumption habit. Most of the patients had tumor size.
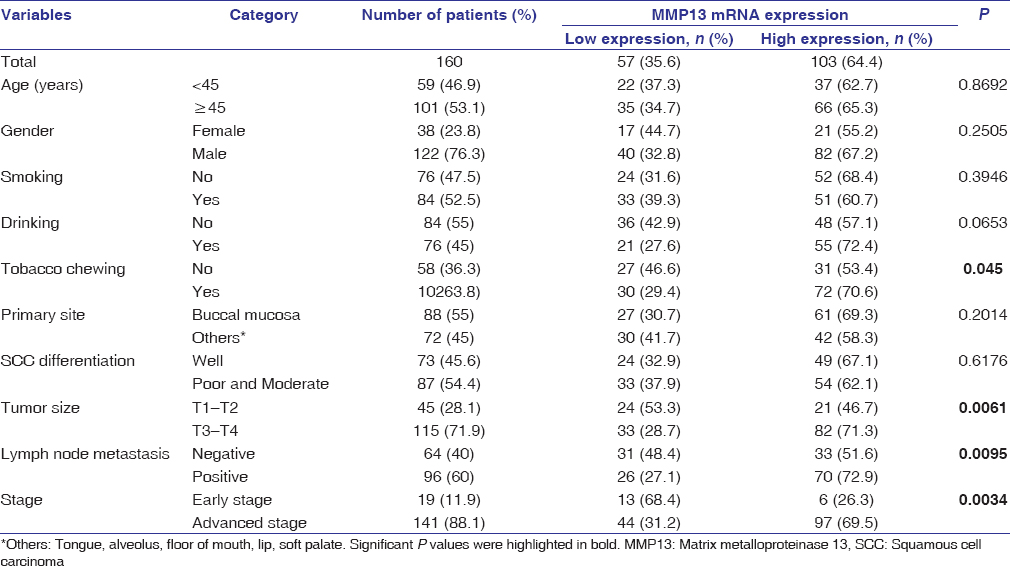 |
Table 1: Correlation of matrix metalloproteinase 13 gene expression with the clinicopathological characteristics Click here to view |
The qRT-PCR analysis revealed highly significant (P < 0.001) induction in the mRNA expression of MMP13 in OSCC patients as compared to matched controls [Figure 1].
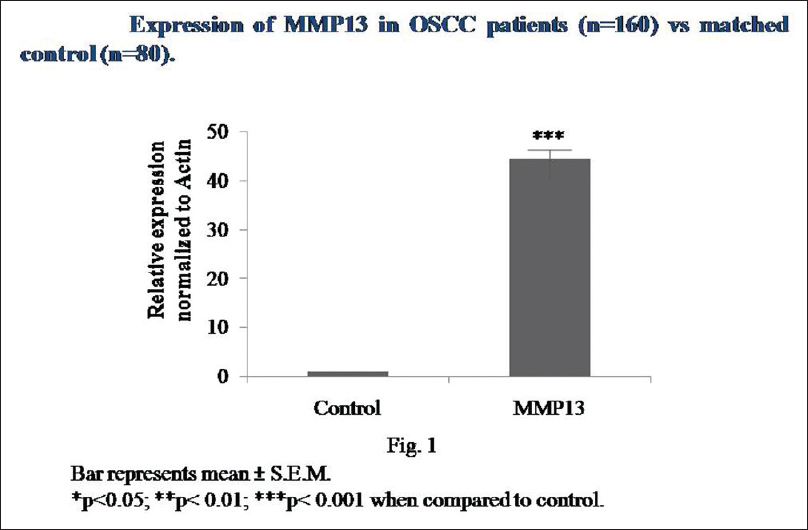 |
Figure 1: MMP13 mRNA expression in OSCC patients and controls Click here to view |
Expression of matrix metalloproteinase 13 mRNA in clinicopathological characteristics
Overexpression of MMP13 mRNA was significantly correlated with chewing habit (P < 0.01), advanced tumor size (T3 and T4, P < 0.001), advanced stage and lymph node metastasis (P < 0.01) in OSCC patients [Table 1].
Association of matrix metalloproteinase 13 mRNA expression with the radiation response
Out of 160 patients, 92 (57.5%) patients responded to radiotherapy (complete response and partial response) and 68 (42.5%) were nonresponders (no response and progressive disease) [Table 2]. The expression of MMP13 mRNA was found to be significantly higher (P < 0.001) in nonresponders as compared to responders.
| Table 2: Association of matrix metalloproteinase-13 gene expression with the radiation response Click here to view |
qRT-PCR analysis further revealed a highly significant (P < 0.001) expression of MMP13 mRNA in patients who were tobacco chewers, along with smoking and alcohol consumption habits, as compared to patients who were tobacco chewers or alcoholic in both nonresponders and responders [Figure 2]. The MMP13 mRNA expression was highly significant in males who were tobacco chewers, alcoholics, and smokers in both responders and nonresponders; however, a significant association of MMP13 mRNA was only found in responder female patients who were only tobacco chewers [Table 3] and [Table 4].
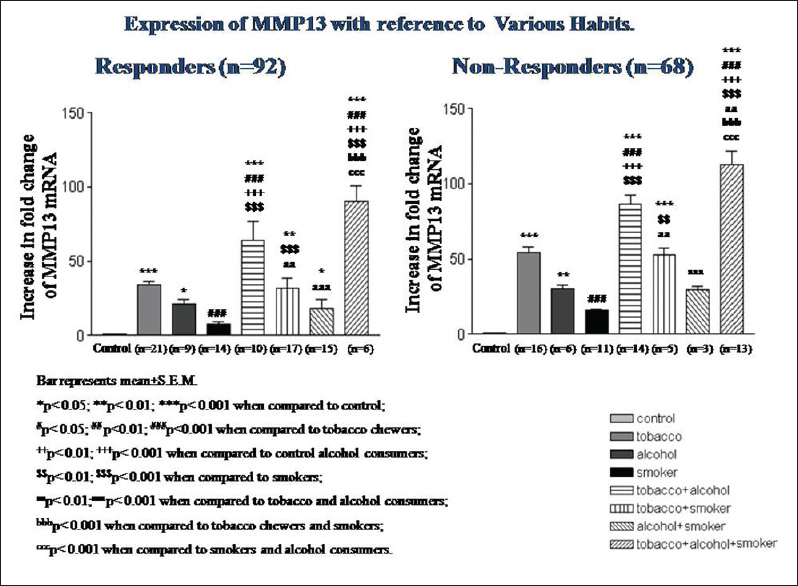 |
Figure 2: Matrix metalloproteinase 13 mRNA expression according to the consumption of tobacco (chewer and smoker) and alcohol Click here to view |
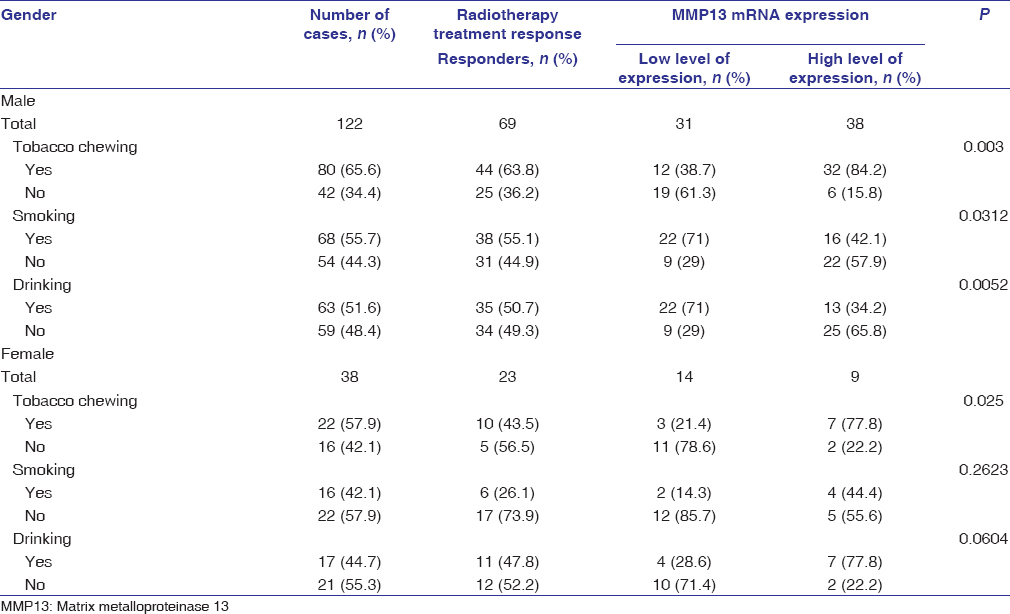 |
Table 3: Correlation of matrix metalloproteinase 13 mRNA expression radiotherapy treatment responders (males and females) Click here to view |
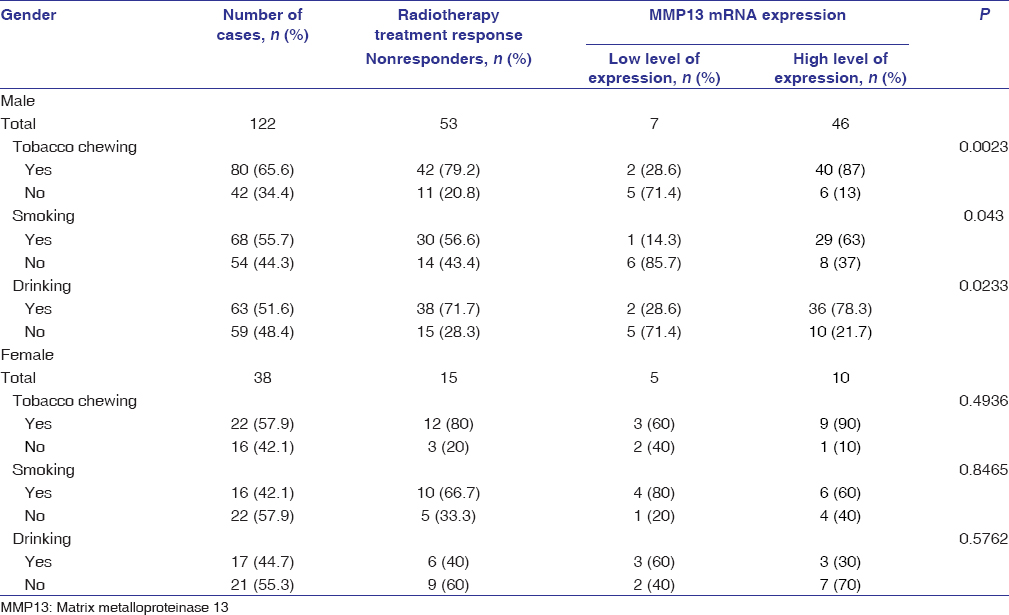 |
Table 4: Correlation of matrix metalloproteinase 13 mRNA expression radiotherapy treatment nonresponders (males and females) Click here to view |
The overexpression of MMP13 mRNA was found to be highly significant in early T-stage (T1, T2) and advanced T-stage (T3, T4) of nonresponders as compared to responders [Figure 3]. Similarly, highly significant increase in MMP13 mRNA expression was also found in both lymph node positive and negative nonresponders as compared to responders [Figure 4].
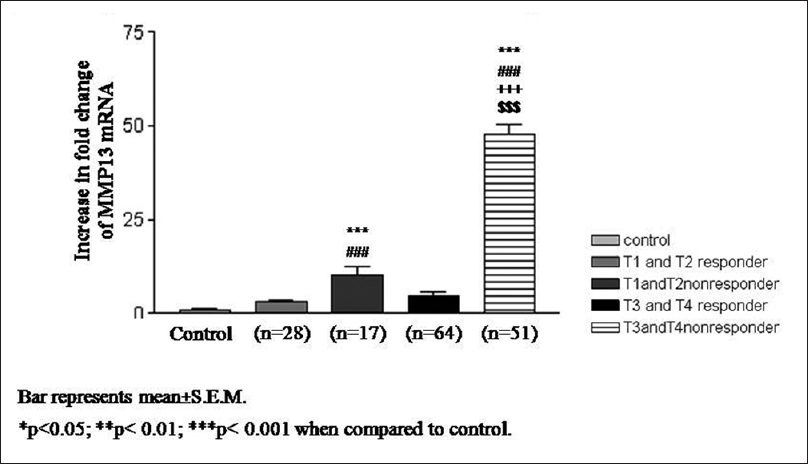 |
Figure 3: Matrix metalloproteinase 13 mRNA expression according to different T-stages of oral squamous cell carcinoma Click here to view |
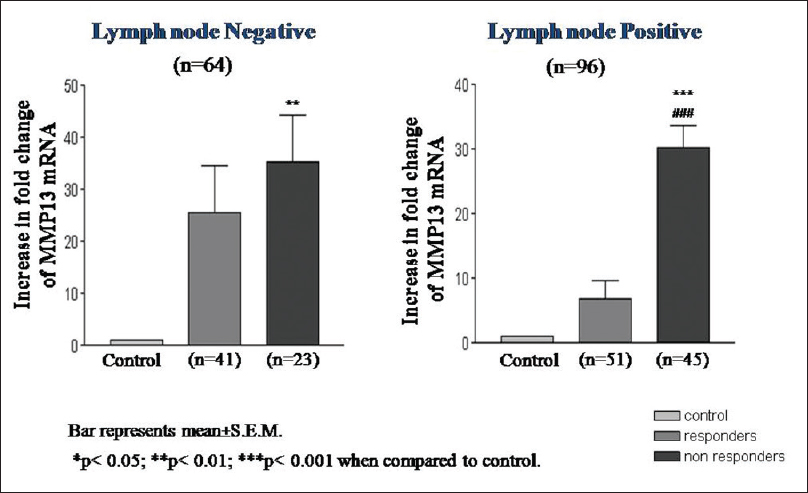 |
Figure 4: Matrix metalloproteinase 13 mRNA expression according to lymph nodes status of oral squamous cell carcinoma Click here to view |
Discussion
Our hypothesis was that MMP13 might be overexpressed in OSCC and its expression could be correlated with clinicopathological parameters and radiation response. The significant expression of MMP13 was found in previous studies in different types of human cancer such as breast cancer, squamous cell carcinomas of the head and neck and vulva, malignant melanomas, chondrosarcomas, and urinary bladder cancer.[7],[8],[9],[10],[11] Several studies demonstrated that MMP13 was overexpressed in oral cancer.[12],[13] Similarly, this study also found the significant increase in the mRNA expression of MMP13 in OSCC patients as compared to matched controls.
Recently, Vincent-Chong et al. demonstrated that overexpression at protein level and mRNA level of MMP3 was found in OSCC patients but only at protein level of expression showed significant association with lymph node metastasis and tumor staging.[12] In the current study, we observed the significant overexpression of mRNA of MMP13 in tobacco chewers, tumor size, advanced stage, and lymph node metastasis. This observation reflects the mRNA level activity of MMP13 which may be involved in the tumor progression of OSCC.
We also found that the significant increase in mRNA expression of MMP13 was found to be more in OSCC patients, who were tobacco chewers, smokers, and alcohol consumers when compared with matched controls.
Several genomic-based studies demonstrated that significantly higher expression of MMP13 was found in advanced stage of OSCC patients.[12],[13] The present findings are in accordance with the previous study demonstrating increase in the expression MMP13 gene in advanced stage.
Currently, radiation therapy has played an imperative constituent in controlling tumor growth and providing cure for patients with OSCC. However, radiotherapy is sometimes ineffective against cancer cells which show resistance to radiation.[14],[15] A cell line-based study demonstrated that this gene was reported to be highly expressed in radio-resistant cells when compared to radiosensitive cells in OSCC.[14] Our data demonstrated increase in the expression of MMP13 to be associated with radio-resistant in OSCC patients.
Our results also revealed that MMP13 gene was also significantly expressed in early as well as advanced stage of OSCC patients who were nonresponders after radiotherapy treatment. The overexpression of MMP13 mRNA levels in nonresponders may further provide support that MMP13 is involved in the invasion and progression of OSCC.
No previous studies have evaluated association between clinicopathological parameters and radiotherapy treatment response in OSCC patients. This gene may help in finding the pattern of radio-resistance in OSCC cases on the basis of substance abuse, lymph node metastasis, and stage of the tumor.
Conclusion
We observed a significant increase in the mRNA expression of MMP13 in OSCC patients as compared to matched controls. Overexpression of MMP13 gene was found to be significantly higher in advanced stage when compared to early stage. The patients who had lower mRNA expression responded better to radiation treatment as compared to a patient who had higher mRNA expression. These results may lead to the consideration that altered level of this gene may be helpful in predicting radiotherapy treatment response in OSCC patients. These results advance our knowledge involving this gene in the resistance to the response of radiation therapy and could be used as potential marker for the radiotherapy treatment. However, larger studies are required to understand the role of MMP13 expression, its predictive significance for radiotherapy outcome in OSCC patients and incorporation of this in the routine clinical practice.
Financial support and sponsorship
This study was financially supported by the Indian Council Medical Research, New Delhi, India.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. |
| Figures |
[Figure 1], [Figure 2], [Figure 3], [Figure 4]
| Tables |

