Khetbadei Lysinia Hynniewta Hadem1, Rajeshwar Nath Sharan2, Lakhan Kma3
1 Cancer and Radiation Countermeasures Unit; Radiation and Molecular Biology Unit, Department of Biochemistry, North-Eastern Hill University, Shillong, Meghalaya, India
2 Radiation and Molecular Biology Unit, Department of Biochemistry, North-Eastern Hill University, Shillong, Meghalaya, India
3 Cancer and Radiation Countermeasures Unit, North-Eastern Hill University, Shillong, Meghalaya, India
| Date of Submission | 08-Jan-2014 |
| Date of Acceptance | 03-Mar-2014 |
| Date of Web Publication | 30-May-2014 |
Correspondence Address:
Lakhan Kma
Cancer and Radiation Countermeasures Unit, North-Eastern Hill University, Shillong, Meghalaya
India
Source of Support: UGC, India project grants to RN Sharan and L Kma. Rajiv Gandhi National Fellowship, UGC, India to KLH Hadem., Conflict of Interest: None
DOI: 10.4103/1477-3163.133520
Abstract
Context: Aristolochia tagala (AT) and Curcuma caesia (CC) have been used traditionally by local herbal practitioners for cancer treatment and as chief ingredients of many polyherbal formulations for various types of ailments. However, there is void in scientific study to evaluate their anti-cancer property. Aims: The aim of this study was to evaluate the anti-carcinogenic properties of the crude methanolic extracts of roots of AT and rhizomes of CC in BALB/c mice exposed to a hepatocarcinogen, diethylnitrosamine (DEN). Settings and Design: (I) Toxicity of herbal plant extracts (HPE); (II) Anticancer studies; (III) Histological studies; and (IV) Biochemical studies. Materials and Methods: To evaluate the effects of these two HPE either alone or following DEN exposure, serum transaminases (aspartate aminotransferase [AST], alanine aminotransferase [ALT]), alkaline phosphatase (ALP), and cancer marker enzyme acetylcholine esterase (AChE) were assayed in mice. In addition, histological study was also carried out under similar conditions. The antioxidant potentials of the HPE were evaluated by monitoring the activity of antioxidant enzymes and metabolites, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione (GSH). Statistical Analysis Used: Statistical analysis was performed by GraphPad Prism 6 Software using one-way analysis of variance followed by the Tukey’s multiple comparisons test. Significance was set at P < 0.05. Results: Our findings show that DEN administration elevated AST, ALT, ALP, and AChE activities. CC or AT extracts attenuated the increased activities of these marker enzymes. The activities of antioxidant enzymes, which were decreased following DEN administration, were significantly increased in mice treated with CC or AT. Conclusions: The present study clearly documents anticarcinogenic and antioxidant properties of AT and CC in DEN-induced mouse liver cancer in vivo.
Keywords: Antioxidant, Aristolochia tagala, cancer, Curcuma caesia, diethylnitrosamine, liver.
| How to cite this article: Hadem KH, Sharan RN, Kma L. Inhibitory potential of methanolic extracts of Aristolochia tagala and Curcuma caesia on hepatocellular carcinoma induced by diethylnitrosamine in BALB/c mice. J Carcinog 2014;13:7 |
| How to cite this URL: Hadem KH, Sharan RN, Kma L. Inhibitory potential of methanolic extracts of Aristolochia tagala and Curcuma caesia on hepatocellular carcinoma induced by diethylnitrosamine in BALB/c mice. J Carcinog [serial online] 2014 [cited 2021 Oct 18];13:7. Available from: https://carcinogenesis.com/text.asp?2014/13/1/7/133520 |
Introduction
Carcinogenesis is a complex process in which a large number of factors interact to disrupt normal cell growth and its controls. In spite of medical advancements, it remains one of the most prominent and challenging killer diseases of humans across the globe accounting for one human death every 5 s or over 13% of all human deaths. [1] The serious toll on human life calls for renewed and urgent efforts to combat the disease. With successful utilization of few natural phytochemicals into mainstream cancer chemotherapy, expectations are high that more medicinal plants would provide much needed therapeutic avenues against human cancer with minimal toxicity and side-effects. In Meghalaya, several traditional practitioners used different herbal preparations for treatment of cancer and other pathophysiological conditions. Among them, two herbal plants are very widely used which are the main subjects of our study in this piece of work.
Aristolochia tagala Cham. (AT; local name – Kur thlong) belong to the family Aristolochiaceae and is included among the 850 species of medicinal plants found in Meghalaya, India. [2] Traditionally, the roots of this plant are used in the form of paste for the treatment of the tumor and as the chief ingredient of many polyherbal formulations for treatment of snake bites, fever, stomach ache, bilious disorder, swollen limb, menstruation problem, rheumatoid pain, etc. Although scientific validation is awaited for most of its therapeutic efficacies, there are report that different parts of AT act as antimicrobial, antiproliferative, cytotoxic, analgesic, antioxidant and insecticidal agent. [3] The leaf and stem extracts were shown to exhibit antiproliferative and cytotoxic activities in vitro. [4]
Curcuma caesia Roxb. (CC; local name – Shyrmit iong) is a member of the Zingiberaceae family and is commonly known as black turmeric. This is a perennial herb found throughout the Himalayan region, North-East and Central India. The rhizomes of this plant are used by local herbal practitioners for cancer treatment as well as for skin diseases, such as boils and carbuncles, inflammation, and gout. Ethno-medicinal uses of the rhizomes of CC for the treatment of cough, asthma, etc., have been reported. [5] CC is shown to possess anti-inflammatory, anti-asthmatic and wound healing properties [6] and is effective against stomach ache, dysentery, and fever. [7] It has also been shown that the crude extract of CC possesses high potential antioxidant properties. [8],[9] It is obvious that both AT and CC are important plants in traditional medicine. However, there is void in scientific study to evaluate their anti-cancer property.
The present study is being carried out to analyze the chemopreventive and/or anti-cancer effects of AT and CC in an in vivo model exposed to an established carcinogen diethylnitrosamine (DEN), which has widely been used in an animal model to induce hepatocarcinogenesis. [10],[11] Enzymes that act as markers for tissue damage because of their tissue specific activity were monitored without or with the herbal plant extract (HPE) exposure following DEN administration. In addition, the modulatory effect of these HPE on the activities of components of antioxidant defense system, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione (GSH) were also analyzed after exposure to DEN.
Materials and Methods
0Chemicals
DEN, acetylthiocholine iodide, 5-5′-dithiobis-(2-nitro benzoic acid) (DTNB), xanthine oxidase, cytochrome c, glutathione reductase, reduced GSH were purchased from Sigma Chemical Co., USA. Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) kits were purchased from Crest Biosystems, India. Jung freezing medium was obtained from Leica Microsystems, China. All other chemicals were of analytical grade procured from various local sources.
Preparation of HPE
AT was identified by Dr. R. Gogoi, Botanical Survey of India, Arunachal Pradesh Regional Center, Itanagar, India, by comparing the voucher specimen 18721, Botanical Survey of India, Shillong and CC, was identified by Dr. P. B. Gurung, North-Eastern Hill University, Shillong, India. The specimen was deposited in the herbarium of the Department of Botany, North-Eastern Hill University, Shillong with voucher specimen ‘NEHU 11927’. These plants were collected from Ri-Bhoi area of Meghalaya, India. The rhizomes of CC and roots of AT were separated, washed and cut into pieces. They were dried in an oven at 37-40 °C for 8-10 days. They were then powdered and methanolic extract was prepared by soaking in 4:1 (v/v) methanol: water (100 g in 250 ml) and stirring continuously overnight at room temperature. The CC and AT extracts were then filtered and the filtrate was evaporated to dryness using a rotary vacuum evaporator. It was finally lyophilized to a yellowish brown powdered, which was dissolved in sterile normal saline at a concentration of 1 g powder/10 ml and centrifuged at 2000 g for 15 min to obtain a clear supernatant. The supernatant, designated as HPE, was used for the experimental purposes.
Preparation of DEN
DEN (density: 0.95 g/ml) was used to induce hepatocellular carcinoma (HCC) in mice. It was prepared in Millipore water and administered intravenously at a dose of 10 mg/kg body weight (bw).
Experimental animals and experimental protocols
The Institutional Ethics Committee of North-Eastern Hill University approved the experimental protocols. Swiss albino BALB/c mice, aged 6-8 weeks from inbred colony, maintained in an animal house 20 °C ± 2 °C and 12 h light and 12 h dark conditions. They were provided with a standard mouse pellets and drinking water ad libitum and used in all experiments. The general scheme of administration of DEN and HPE to mice is schematically shown in [Figure 1]a. DEN was administered by intravenous (i.v.) route [11],[12] at weekly intervals from week zero (start of the experiment) until the end of experiment (16 or 28 weeks). The exposures to the HPE (either AT or CC) were initiated from week 10 until the end of experiment (weeks 16 or 28) by thrice weekly intraperitoneal (i.p.) injections.
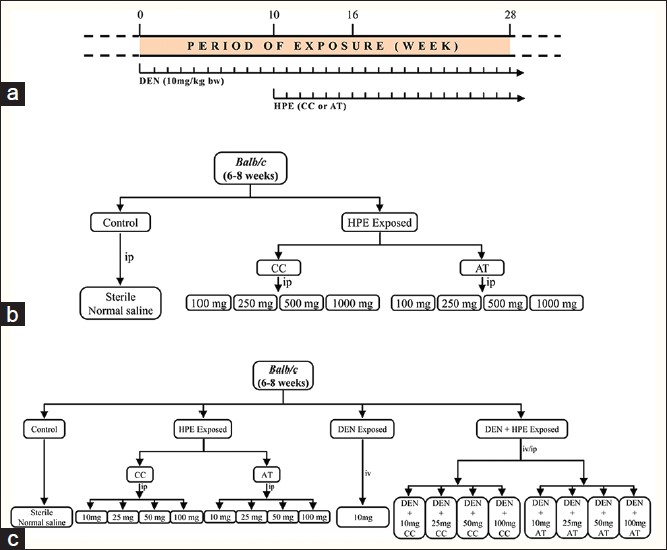 |
Figure 1: Schematic diagram to show dose scheduling and exposure protocol (a) and experimental design of toxicity studies (b) and anticancer studies (c) of Curcuma caesia and Aristolochia tagala in diethylnitrosamine exposed Swiss albino BALB/c mice Click here to view |
Experimental design
Toxicity of HPE
0The toxicity of HPE as well as effects of HPE on the behavior of mice was determined according to the method of Mendes et al. [13] Mice were divided into nine groups of eight mice each to assess the toxicity of the HPE. CC and AT extracts were administered by i.p. injection at doses of 100, 250, 500, and 1000 mg/kg bw. The control group mice received sterile saline injections only [Figure 1]b. Toxicity and mortality were recorded for up to seven days. Biochemical alterations induced by CC or AT were analyzed in serum after 24 h of exposure.
Anticancer studies
Mice were divided into different groups, namely, control, DEN exposed, HPE exposed (varying doses of both the plant extracts) and DEN + HPE exposed groups (varying doses of both plant extracts) [Figure 1]c. Control mice received sterile saline injections only. The mice in DEN exposed group received weekly i.v. injections of DEN (10 mg/kg bw) each for the total duration of the experiment [Figure 1]c. The HPE exposed mice, which served as a positive control group, were subdivided into eight cages. They received i.p. injections of CC or AT thrice a week from tenth week onwards at 10, 25, 50, and 100 mg/kg bw [Figure 1]c. The mice in the DEN + HPE exposed group were exposed to DEN as above and in addition, also given i.p. injection thrice weekly either by CC or AT from 10 th week onwards at doses of 10, 25, 50, and 100 mg/kg bw [Figure 1]c. Data were obtained as final mean value from three independent replicating sets of mice. An average of 5-8 mice was used in each group for each set. The mean of separate experiments in each set was calculated separately. The experiments were repeated under identical condition in the second and third sets and the mean was obtained in the similar manner for each group. For rigorous statistical correctness, the final mean of all the mean values from three sets in a group was calculated and used as the final data shown in figures. Similar strategy was used for the studies at each dose as indicated in [Figure 1]c unless indicated otherwise.
Histological studies
Livers of all the experimental groups [Figure 1]c and corresponding regions of liver of age-matched control mice were removed, washed in saline, and then embedded in Junk medium according to the method describe by Fischer. [14] Sections were prepared and stained with hematoxylin and eosin. The tissues were then examined microscopically.
Biochemical studies
Blood was collected by retro-orbital bleeding and centrifuged at 2000 g for 20 min at 4 °C. ALT, AST, and ALPs activities were determined in the serum using the enzyme assay kit according to the manufacturer’s instructions using the Blood Analyzer 3000. For enzyme assays in the liver, it was perfused by standard method and 0.25 g tissues were homogenized with 2.5 ml of 0.25 M sucrose solution. The whole homogenate was centrifuged at 20,000 g for 30 min at 4 °C. Supernatant was assayed for acetylcholine esterase (AChE), SOD, CAT, and GPx. [15],[16],[17],[18] Briefly, for AChE activity assay, 2800 μl phosphate buffer (100 mM, pH 7.4), 50 μl AcSCHI (50 mM) and 100 μl DTNB (150 mM) was taken in a 3 ml cuvette with a light path of 1 cm and mixed thoroughly. The reaction was started by adding 50 μl supernatant and the increase in absorbance 1/min at 30 °C was recorded at 412 nm. For SOD activity assay, 2530 μl phosphate buffer (50 mM, pH 7.8), 300 μl xanthine (0.5 mM), 100 μl cytochrome c (0.1 mM) and 20 μl supernatant were taken and mixed thoroughly in a 3 ml cuvette with a light path of 1 cm. The reaction was initiated by adding 50 μl xanthine oxidase (0.2 U) and change in absorbance 1/min at 25 °C was recorded at 550 nm. For CAT activity assay, 2300 μl phosphate buffer (50 mM, pH 7.0) and 680 μl H 2 O 2 ( 30 mM) were mixed thoroughly in a 3 ml quartz cuvette with a light path of 1 cm. The reaction was initiated by adding 20 μl supernatant and decrease in absorbance was monitored at 25 °C for a time interval of 15 s for 1 min at 240 nm. For GPx activity assay, 100 μl phosphate buffer (250 mM, pH 7.0), 100 μl GSH (10 mM), 100 μl glutathione disulfide (GSSG) red (2 U), 100 μl nicotinamide adenine dinucleotide phosphate (NADPH) (2.5 mM) and 20 μl supernatant were mixed thoroughly in a 1 ml quartz cuvette with a light path of 1 cm and incubated at 37 °C for 10 min. The reaction was initiated by adding 100 μl of H 2 O 2 ( 12 mM). The linear decrease in NADPH was monitored at 366 nm change in absorbance 1/min was recorded. The specific activity of these enzymes was calculated and expressed as U/mg protein.
GSH was estimated according to Owens and Belcher. [19] Briefly, 10 μl of supernatant was added to a mixture of 1570 μl phosphate buffer (100 mM, pH 7.0) and 20 μl of DTNB (1.5 mg/ml), mixed thoroughly and absorbance was read at 412 nm after 3 min. The amount of GSH present in the sample was compared from triplicate standards containing 5 μg of GSH per 500 μl of buffer. The results were expressed as mg/g of tissue. Total proteins were determined by the method of Bradford [20] using bovine serum albumin as a standard.
Data and statistical analysis
Statistical analysis was performed by GraphPad Prism 6 Software 1995-2014 GraphPad Software, Inc., USA, using one-way analysis of variance followed by the Tukey’s multiple comparisons test. Data have been presented as mean ± standard deviation. Significance was set at P < 0.05.
Results
0PE induced toxicity
Mice did not show any abnormalities/stress, such as circling, labored breeding, abdominal contractions or mortality after administration of CC or AT (HPE) up to the dose of 1000 mg/kg bw.
ALT activity of mice did not show any significant change up to 500 mg/kg bw dose of CC exposure. However, there was a significant (P < 0.01) increase in its activity at 1000 mg/kg bw. Upon exposure to AT, on the other hand ALT enzyme activity fluctuated widely at 250, 500, and 1000 mg/kg bw [Figure 2]a. AST activity exhibited a trend of increase with higher doses of CC and AT, that is, 500 mg/kg bw onwards [Figure 2]b. A similar trend was also observed for ALP activity of mice administered with CC or AT with strong inhibitory effect at the highest dose of 1000 mg/bw onwards [Figure 2]c.
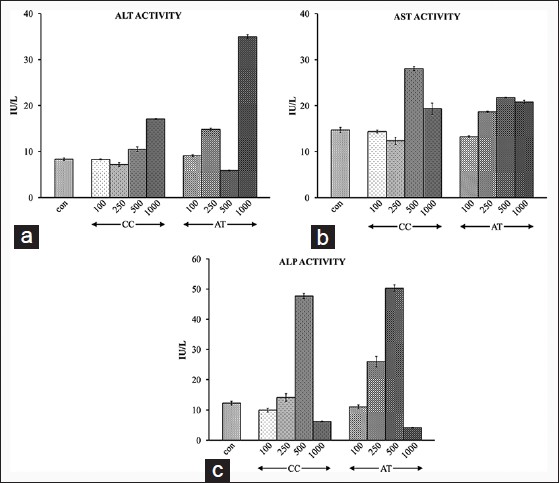 |
Figure 2: Alanine aminot rans ferase (a), a spar tate aminotransferase (b), and alkaline phosphatase (c) activity after 24 h of i.p. administration of different doses of 100, 250, 500, and 1000 mg/kg bw of Curcuma caesia and Aristolochia tagala Click here to view |
Effects of HPE and DEN exposures on the body and liver weights
Mice exposed to DEN showed a significant (P < 0.01) decrease in their bws when compared to age-matched control mice at both 16 and 28 weeks [Table 1]. In contrast, the liver weight of DEN exposed was significantly (P < 0.01) higher at both 16 and 28 weeks [Table 1]. Exposure of mice to DEN along with either CC or AT exhibited weak trend of recovery of body and liver weights.
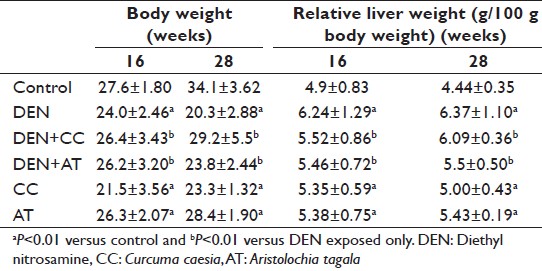 |
Table 1: Body and liver weights at 16 and 28 weeks under different experimental conditions Click here to view |
Effects of HPE and DEN exposures on the morphology of liver
DEN exposure caused appearance of preneoplastic nodules in liver at 16 weeks, which increased in size and number at 28 weeks [Figure 3]b. The nodule incidence was also significantly higher at 28 weeks (P < 0.01) [Table 2]. The periphery of the liver was also seen to be irregular [Figure 3]b. This change in morphology was seen to revert close to control with fewer nodules in the DEN + CC (P < 0.01) [Figure 3]c, [Table 2] or DEN + AT [Figure 3]d groups. Exposure to either HPE did not cause any observable change in the liver morphology [Figure 3]e and f, [Table 2].
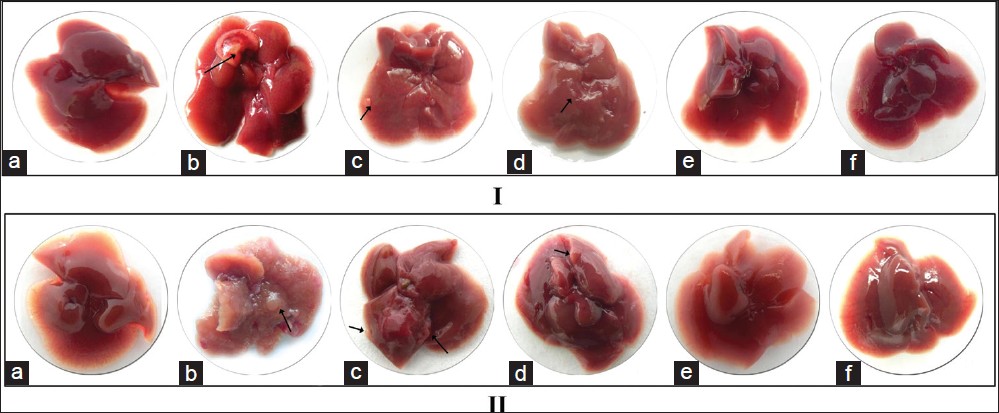 |
Figure 3: Morphology of mice liver at 16 weeks (I) and 28 weeks (II) under different experimental conditions. (a) Control, (b) diethylnitrosamine (DEN)-exposed, (c) DEN + Curcuma caesia (CC) exposed, (d) DEN + Aristolochia tagala (AT) exposed, (e) treated with CC alone and (f) treated with AT alone. Arrow indicates nodule formation Click here to view |
 |
Table 2: Nodule incidence and number of nodules at 16 and 28 weeks under different experimental conditions Click here to view |
Effects of HPE and DEN exposures on histology of liver
The HE stained sections of liver from control mice revealed well-defined symmetrical, mono-nucleated liver cells with a regular outline and close contact with the neighboring cells [Figure 4]a. DEN exposed liver sections showed more irregular cells and fragmented nuclei, which was more pronounced at 28 weeks. Nuclei also appeared to have lost their spherical appearance [Figure 4]b. However, sections of liver from DEN + CC [Figure 4]c or DEN + AT [Figure 4]d exposed mice appeared to reverse the structural changes induced by DEN exposure. Exposure to CC or AT alone did not induce any significant change [Figure 4]e and f.
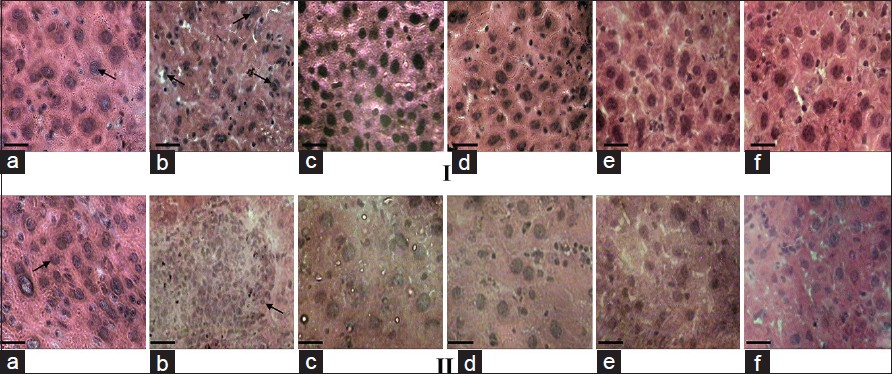 |
Figure 4: H and E histological sections of mice liver tissues at 16 (I) and 28 weeks (II) under different experimental conditions. (a) Control, (b) diethylnitrosamine (DEN)-exposed, (c) DEN + Curcuma caesia (CC) exposed, (d) DEN + Aristolochia tagala (AT) exposed, (e) treated with CC alone and (f) treated with AT alone. Arrows in I and IIa showing cell with normal spherical nucleus with regular outline; ‘Ib’ showing spherical structure of nucleus is not retained with irregular outline. ‘IIb’ showing uncontrolled cell proliferation. Bar = 20 ìm alanine aminotransferase (a), aspartate aminotransferase (b), and alkaline phosphatase Click here to view |
Effects of HPE and DEN exposures on serum transferase and AChE enzymes
Exposure of DEN treated mice to either of the HPE essentially brought down the elevated levels of ALT [Figure 5]a, AST [Figure 5]b, and ALP [Figure 5]c toward its control as observed after 16 and 28 weeks. In the relative sense, the inhibitory effect of CC was more consistent than AT. Treatment with CC or AT alone did not show any significant changes in ALT, AST or ALP activities when compared to control [Figure 5].
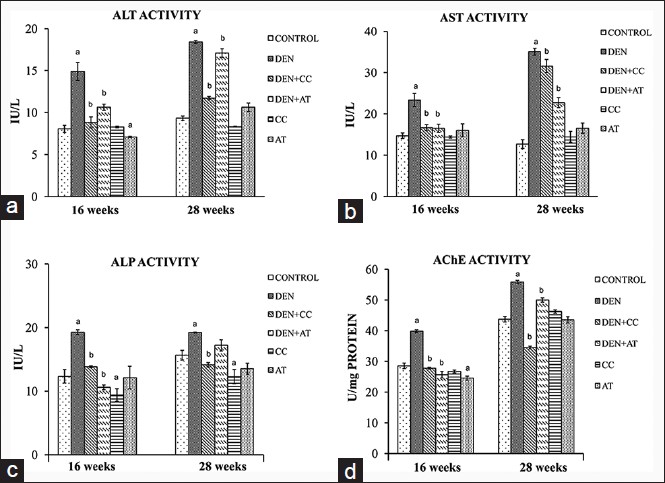 |
Figure 5: Serum transferases and acetylcholine esterase (AChE) enzyme activities of mice at 16 and 28 weeks under different experimental conditions. a-d showing alanine amino transferase, aspartate aminotransferase, alkaline phosphatase, and AChE enzyme activities, respectively. (a): P < 0.05 versus control and (b): P < 0.05 versus diethylnitrosamine exposed only Click here to view |
[Figure 5]d shows the activity of tumor marker enzyme AChE in liver. A significant increased (P < 0.001) was seen in DEN exposed compared to normal control mice at both 16 and 28 weeks. DEN + CC and DEN + AT groups showed a significant rescue of AChE to almost control level.
Effects of HPE and DEN exposures on antioxidant enzymes and GSH levels
[Figure 6]a-c shows the levels of SOD, CAT and GPx in normal control and treated mice. DEN exposed mice showed a significant decrease in all these antioxidant enzymes studied in comparison to normal control mice. Mice groups exposed to DEN + CC and DEN + AT showed a recovery in the activities of all three enzymes at 16 as well as 28 weeks. Similarly, decrease in the GSH levels in DEN exposed mice was recovered by treatment with either of HPE [Figure 6]d.
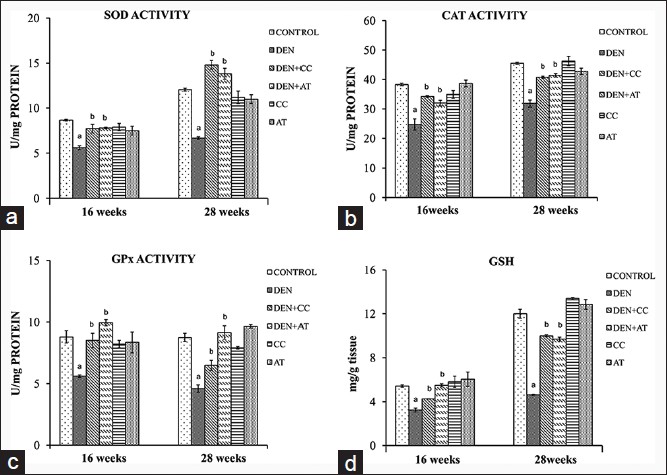 |
Figure 6: Antioxidant enzymes and glutathione (GSH) levels of mice at 16 and 28 weeks under different experimental conditions. a-d showing superoxide dismutase, catalase, glutathione peroxidase activities and GSH levels, respectively. (a): P < 0.05 versus control and (b): P < 0.05 versus diethylnitrosamine exposed only Click here to view |
Discussion
Carcinogenesis is a general term used to denote the development of neoplasia. Carcinogenesis may be actively induced in living organisms by a variety of different agents that are generally chemical or physical in nature. [21] Carcinogenesis by chemicals involves either a direct action of the chemical on cellular deoxyribonucleic acid (DNA) or metabolism of the parent chemical to an active or ultimate form, which can then react with cellular DNA or enzymes involved in DNA repair to produce a permanent chemical change in the DNA structure. [22] DEN is a well-known hepatocarcinogen normally used to induce liver cancer in animal models. It has been shown that on primary metabolic activation, DEN produces the promutagenic adducts, O 6 -ethyl deoxyguanosine and O 4 and O 6 -ethyl deoxythymidine that can produce DNA chain damage, depurination or binding to DNA and often generating a miscoding gene sequence, paving a way to the initiation of liver carcinogenesis. [23],[24] A mouse model to study dimethylnitrosamine induced carcinogenesis in mice is established in our laboratory. [11,25] The pathological changes during the progression of tumors and its inhibition are expected to be reflected in the biological and histological parameters of the host system.
One of the symptoms associated with hepatocellular carcinoma is weight loss and tissue wasting. [26] There was a sharp decrease in the bw and an increased in the liver weights at both 16 and 28 weeks in animals exposed to the hepatocarcinogen DEN [Table 1]. Though it was expected that treatment with the two extracts would reverse the trend we did not record any modulation of the body and liver weights by treatment with CC and AT.
Enzymes are essentially harbored inside cells of their origin and restrained within the plasma membrane. Distress such as exposure to the hepatocarcinogen DEN can cause the plasma membrane to deteriorate and lead to hepatocellular damage. In this case, intracellular enzymes such as transaminases and phosphatases could be released into the plasma. This may lead to varied pattern depending on the variation in clearance of the enzyme and its leakage at various stages of the disease. Aminotransferases, aspartate transaminases and alanine transaminases, are reliable marker enzymes of liver and they are the first enzymes to be used in diagnostic enzymology when liver damage has occurred. [27] The elevations in the transaminases are considered as the most sensitive markers in the diagnosis of hepatocellular damage and loss of functional integrity of the membrane. [28] Elevated serum ALT level correlates with a high incidence of HCC development in patients with chronic hepatitis. [29] The measurement of ALP activity is also useful as an indicator of liver function. [30] In the liver, it is closely connected with lipid membrane in the canalicular zone, so that any interference with the bile flow, whether extrahepatic or intra-hepatic leads to increased serum levels of ACP and ALP activities.
AChE is not present only in excitable tissues, but also in some non-excitable tissues, such as liver, placenta, and blood cells. Many tumors express AChEs, indicating that the enzymes may be functionally important in neoplastic cell transformation. Studies have shown that ChE genes are amplified in leukemias and ovarian carcinomas [31] structurally altered or abnormally expressed in the brain and nonbrain neoplasm, [32] the protein product encoded by ChE genes also shows abnormal properties in meningiomas, glioblastomas, bladder carcinomas and others. [33],[34] Since AChE is a membrane anchored enzyme and cancer induction is accompanied by membrane changes it is released in the supernatant obtained from liver tissues of the experimental mice were monitored. Thus, AChE serve as a marker to monitor the induction of carcinogenesis in liver. DEN administration showed a significant increase in serum transaminases, serum ALP, and AChE activities due to over production of these enzymes in tumor cells, which might have caused an increase in the permeability of the cell membrane resulting in the liberation of this enzyme into serum. [35] CC and AT treatments attenuated the increased activities of these marker enzymes caused by DEN induction in the animals. This might be due to the ability of these extracts to uphold parenchymal cell regeneration in liver, by repairing hepatic tissue damage caused by tumor induction, thus protecting membrane integrity and thereby decreasing enzyme leakage. [36],[37],[38]
Specific antioxidant enzymes have been designed by nature to destroy superoxide and hydroperoxides. The advantage of using enzymes is that the steady-state concentration of peroxides can be adapted to cellular requirements; several of the antioxidant enzymes can be induced, inhibited or activated by endogenous effectors, [39] and they play an important role in the regulation of metabolic pathways and specific functions of aerobic cells. Enzymatic degradation of superoxide is ensured by SODs, while that of hydroperoxides is ensured by CAT and GPx. Superoxide anion (O -2) can be dismutated to hydrogen peroxide (H 2 O 2 ) by the enzyme SOD and generated H 2 O 2 can be quenched by GPx in mitochondria, or by CAT in the cytosol. In a variety of malignancies, elevated lipid peroxidation is associated with reduced activity of antioxidants. Deficiency of SOD and CAT results in decreased detoxification of oxygen radicals, which leads to attack of OFR/ROS on protein and nucleic acids. Hence, the activities of these enzymes are decreased in cancerous condition. [40-43] Such studies substantiate with our findings as there was a significant depletion in the activities of antioxidant enzymes in livers of animals treated with DEN when compared with normal animals. This indicates the severity of the oxidative stress during DEN metabolism, which could have inhibited the activities of these enzymes.
GSH is the major nonenzymatic component of intracellular antioxidant defenses it works either as a nucleophile for efficient detoxification of reactive electrophiles, or as an antioxidant. [44] GSH is present in a reduced, biologically active form (GSH), and in an oxidized form, namely GSSG. [45] The glutathione redox system is balanced when the GSH-to-GSSG ratio is high. Almost 90% of the total GSH is maintained in the reduced form through de novo GSH synthesis, enzymatic reduction of GSSG by GSSG reductase, and uptake of exogenous GSH. [45] The GSH system acts to maintain an appropriate intracellular redox homeostasis. During the reaction of H 2 O 2 scavenging, GSH is oxidized to GSSG by the enzyme GPx [46] and GR reduces GSSG to GSH. [47] The level of non-enzymic antioxidant GSH decreases in hepatoma bearing animals. [48] The results of the present study also correlate with such findings. It might be due to over utilization of these antioxidants to scavenge free radicals.
In the present study, the chemopreventive activity of both the extracts was further substantiated by morphology with the appearance of preneoplastic nodules, which is advanced at 28 weeks. Morphology of mice induced with DEN and treated with CC and AT showed a fewer nodules. The nodules that developed in liver of DEN exposed mice were confirmed by histological examination. Histology showed a rapid increased in cell division and showed features of HCC, [49] whereas treatment with CC and AT shows a close resemblance to the control.
Conclusion
The present investigation attempts to highlight the chemopreventive potential of CC and AT against DEN-induced HCC by enhancing antioxidant status through free radical scavenging mechanism and having potential of protecting endogenous enzymatic and nonenzymatic antioxidant activity. Preliminary analysis of these plants has shown that these plants contain bioactive compounds with antioxidant properties (unpublished results). It is also suggested that CC and AT possess some anti-cancer properties and are promising candidate natural medicinal products for further studies/evaluation as anti-cancer drugs. Further studies pertaining to possible mechanism of action of these extracts against DEN-induced liver cancer and identification of the active component of these herbal plants are underway in our laboratory.
Acknowledgment
The authors would like to thank UGC, Government of India for the financial support in the form of project grants to RN Sharan and L Kma, and Rajiv Gandhi National Fellowship to KLH Hadem to carry out the work.
References
| 1. | IARC, WHO Cancer. Lyon, France: World Health Organization. [Last reviewed on 2014 Feb 3].  |
| 2. | Singh AK. Probable agricultural biodiversity heritage sites in India: V. The Garo, Khasi, and Jaintia Hills Region. Asian Agrihist 2010;14:133-56.  |
| 3. | Dey A, De JN. Pharmacology and medicobotany of Aristolochia tagala Cham: A review. Pharma Sci Monit 2012;3:110-22.  |
| 4. | Garg A, Darokar MP, Sundaresan V, Faridi U, Luqman S, Rajkumar S, et al. Anticancer activity of some medicinal plants from high altitude evergreen elements of Indian Western Ghats. J Res Educ Indian Med 2007;13:1-6.  |
| 5. | Kala CP. Ethnomedicinal botany of the Apatani in the Eastern Himalayan region of India. J Ethnobiol Ethnomed 2005;1:11.  |
| 6. | Tushar, Basak S, Sarma GC, Rangan L. Ethnomedical uses of Zingiberaceous plants of Northeast India. J Ethnopharmacol 2010;132:286-96.  |
| 7. | Pfoze NL, Kumar Y, Myrboh B. Survey and assessment of ethnomedicinal plants used in Senapati District of Manipur State, Northeast India. Phytopharmacology 2012;2:285-311.  |
| 8. | Dhal Y, Deo B, Sahu RK. Comparative antioxidant activity of non-enzymatic and enzymatic extracts of Curcuma zedoaria, Curcuma angustifolia and Curcuma caesia. Int J Plant Anim Environ Sci 2012;2:2231-4490.  |
| 9. | Chirangini P, Sharma GJ, Sinha SK. Sulfur free radical reactivity with curcumin as reference for evaluating antioxidant properties of medicinal zingiberales. J Environ Pathol Toxicol Oncol 2004;23:227-36.  |
| 10. | Pitot HC, Sirica AE. The stages of initiation and promotion in hepatocarcinogenesis. Biochim Biophys Acta 1980;605:191-215.  |
| 11. | Pariat T, Sharan RN. Low dose exposure of diethylnitrosamine affects mice liver thymidine kinase. Life Sci 1995;57:2431-7.  |
| 12. | Alam A, Singha LI, Singh V. Molecular characterization of tumor associated antigen in mice exposed to a hepatocarcinogen. Mol Cell Biochem 2005;271:177-88.  |
| 13. | Mendes MM, Vale LH, Lucena MN, Vieira SA, Izidoro LF, Junior RJ, et al. Acute toxicity of Schizolobium parahyba aqueous extract in mice. Phytother Res 2010;24:459-62.  |
| 14. | Fischer AH, Jacobson KA, Rose J, Zeller R. Preparation of cells and tissues for fluorescence microscopy. In: Spector DL, Goldman R, editors. Basic Methods in Microscopy. New York: Cold Spring Harbor Laboratory Press; 2006.  |
| 15. | Ott P, Jenny B, Brodbeck U. Multiple molecular forms of purified human erythrocyte acetylcholinesterase. Eur J Biochem 1975;57:469-80.  |
| 16. | Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys 1986;247:1-11.  |
| 17. | Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121-6.  |
| 18. | Wendel A. Glutathione peroxidase. Methods Enzymol 1981;77:325-33.  |
| 19. | Owens CW, Belcher RV. A calometric micro-method for the determination of glutathione. J Biochem 1964;94:705-13.  |
| 20. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.  |
| 21. | Pitot HC. The molecular determinants of carcinogenesis. Symp Fundam Cancer Res 1986;39:187-96.  |
| 22. | Pitot HC. Fundamentals of Oncology. New York: Marcel Dekker; 1986.  |
| 23. | Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: Bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 1996;71:57-81.  |
| 24. | Dagli MLZ. Agentes antineoplasicos. In: Spinosa HS, Gorniak SL, Bernardi MM, editors. Farmacologia Aplicada a, Medicina Veterinaria. São Paulo, Brasil: Guanabara-Koogan; 2002. p. 577-608.  |
| 25. | Kma L, Sharan RN. In vivo exposure of swiss albino mice to chronic low dose of dimethylnitrosamine (DMN) lowers poly-ADP-ribosylation (PAR) of bone marrow cell and blood lymphocyte proteins. Mol Cell Biochem 2006;288:143-9.  |
| 26. | Kwe MC. Tumors of the liver. In: Zakim D, Boyer TD, editors. Hepatology a Text Book of Liver Disease. Philadelphia: Saunders; 1996. p. 1513-48.  |
| 27. | Whittby LG, Perey-Robb IW, Smith AT. Enzymes tests in diagnosis. In: Lecturer Notes in Clinical Chemistry. London: Black Well Scientific Publications; 1984. p. 138-69.  |
| 28. | Plaa GL, Hewitt WR. Detection and evaluation of chemically induced liver injury. In: Hayes W, editor. Principles and Methods of Toxicology. New York: A Raven Press; 1989. p. 399-628.  |
| 29. | Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, et al. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer 1999;86:589-95.  |
| 30. | Nair KG, Deepadevi KV, Arun P, Kumar VM, Santhosh A, Lekshmi LR, et al. Toxic effect of systemic administration of low doses of the plasticizer di-(2-ethyl hexyl) phthalate [DEHP] in rats. Indian J Exp Biol 1998;36:264-72.  |
| 31. | Zakut H, Ehrlich G, Ayalon A, Prody CA, Malinger G, Seidman S, et al. Acetylcholinesterase and butyrylcholinesterase genes coamplify in primary ovarian carcinomas. J Clin Invest 1990;86:900-8.  |
| 32. | Karpel R, Ben Aziz-Aloya R, Sternfeld M, Ehrlich G, Ginzberg D, Tarroni P, et al. Expression of three alternative acetylcholinesterase messenger RNAs in human tumor cell lines of different tissue origins. Exp Cell Res 1994;210:268-77.  |
| 33. | Zakut H, Even L, Birkenfeld S, Malinger G, Zisling R, Soreq H. Modified properties of serum cholinesterases in primary carcinomas. Cancer 1988;61:727-37.  |
| 34. | Soreq H, Lapidot-Lifson Y, Zakut H. A role for cholinesterases in tumorigenesis? Cancer Cells 1991;3:511-6.  |
| 35. | Ramakrishnan G, Augustine TA, Jagan S, Vinodhkumar R, Devaki T. Effect of silymarin on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Exp Oncol 2007;29:39-44.  |
| 36. | Pawar R, Gopalakrishnan C, Bhutani KK. Dammarane triterpene saponin from Bacopa monniera as the superoxide inhibitor in polymorphonuclear cells. Planta Med 2001;67:752-4.  |
| 37. | Rohini G, Sabitha KE, Devi CS. Bacopa monniera Linn. Extract modulates antioxidant and marker enzyme status in fibrosarcoma bearing rats. Indian J Exp Biol 2004;42:776-80.  |
| 38. | Jadon A, Bhadauria M, Shukla S. Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol 2007;109:214-8.  |
| 39. | Harris ED. Regulation of antioxidant enzymes. FASEB J 1992;6:2675-83.  |
| 40. | Buzby GP, Mullen JL, Stein TP, Miller EE, Hobbs CL, Rosato EF. Host-tumor interaction and nutrient supply. Cancer 1980;45:2940-8.  |
| 41. | Oberley LW, Oberley TD. Free radicals, cancer and aging. In: Johnson JE Jr, Walford R, Harman DM, Alan R, editors. Free Radicals, Aging and Degenerative Diseases. New York: Liss Inc.; 1986. p. 325-71.  |
| 42. | Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. Oxford: Clarendon Press; 1989. p. 543-70.  |
| 43. | Ray G, Batra S, Shukla NK, Deo S, Raina V, Ashok S, et al. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat 2000;59:163-70.  |
| 44. | Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001;30:1191-212.  |
| 45. | Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 2010;48:749-62.  |
| 46. | Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983;52:711-60.  |
| 47. | Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem 1982;257:5751-4.  |
| 48. | Thirunavukkarasu C, Singh JP, Selvendiran K, Sakthisekaran D. Chemopreventive efficacy of selenium against N-nitrosodiethylamine-induced hepatoma in albino rats. Cell Biochem Funct 2001;19:265-71.  |
| 49. | Kushida M, Aiso S, Morimura K, Wei M, Wanibuchi H, Nagano K, et al. Absence of beta-catenin alteration in hepatic tumors induced by p-nitroanisole in Crj: BDF1 mice. Toxicol Pathol 2006;34:237-42.  |
Authors
Ms. Khetbadei Lysinia Hynniewta Hadem: Cancer and Radiation Countermeasures Unit, Radiation and Molecular Biology Unit, Department of Biochemistry, North-Eastern Hill University, Shillong, Meghalaya – 793 022, India.
Prof. Rajeshwar Nath Sharan: Radiation and Molecular Biology Unit, Department of Biochemistry, North-Eastern Hill University, Shillong, Meghalaya – 793 022, India.
Dr. Lakhan Kma: Cancer and Radiation Countermeasures Unit, Department of Biochemistry, North-Eastern Hill University, Shillong, Meghalaya – 793 022, India.
Figures
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6]
Tables
