Ji-Kang Jeong1, Hee-Kyung Chang2, Kun-Young Park1
1 Department of Food Science and Nutrition, Pusan National University, Busan 609-735, Korea
2 Department of Pathology, Kosin University Gospel Hospital, Busan 602-702, Korea
| Date of Submission | 16-Mar-2012 |
| Date of Acceptance | 15-Jul-2012 |
| Date of Web Publication | 30-Aug-2012 |
Correspondence Address:
Kun-Young Park
Department of Food Science and Nutrition, Pusan National University, Busan 609-735
Korea
Source of Support: This work was supported by funds from the National Institute of Agricultural Science and Technology, Rural Development Administration, Korea, Conflict of Interest: None
DOI: 10.4103/1477-3163.100404
Abstract
Backgrounds: Meju is the main ingredient and the starter culture of traditional Korean fermented soybean foods; these fermented soybean products are well-known for their various health benefits, including anticancer effects. We developed the grain-type meju using probiotic mixed starter cultures to improve the qualities and functionalities of fermented soybean products, as well as the meju itself. In this study, the inhibitory effects of the grain-type meju were investigated in azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced colon carcinogenesis mice model. Materials and Methods: AOM and DSS colon carcinogenesis was induced in female C57BL/6 mice and meju was orally administered for 4 weeks. The body weight, colon length, and colon weight of mice were determined, and colonic tissues were histologically observed. The serum levels of proinflammatory cytokines and the levels of inflammation- and apoptosis-related genes in colonic tissue were also analyzed. Results: The administration of meju using probiotic mixed starter cultures ameliorated the symptoms of colon cancer and reduced number of neoplasia, and reduced serum proinflammatory cytokine levels and iNOS and COX-2 expression levels in colonic tissue. It increased Bax and reduced Bcl-2 expression levels and increased p21 and p53 expression in colonic tissues. Conclusion: The meju showed inhibitory effects on the progression of colon cancer induced by AOM and DSS by ameliorating the symptoms of colon cancer, reducing the number of neoplasias and regulating proinflammatory cytokine levels and the expressions of inflammation- and apoptosis-related genes in the colonic tissue.
Keywords: Colon carcinogenesis, Inflammation, Meju, starter culture
How to cite this article:
Jeong JK, Chang HK, Park KY. Inhibitory effects of meju prepared with mixed starter cultures on azoxymethane and dextran sulfate sodium-induced colon carcinogenesis in mice. J Carcinog 2012;11:13
How to cite this URL:
Jeong JK, Chang HK, Park KY. Inhibitory effects of meju prepared with mixed starter cultures on azoxymethane and dextran sulfate sodium-induced colon carcinogenesis in mice. J Carcinog [serial online] 2012 [cited 2021 Oct 14];11:13. Available from: https://carcinogenesis.com/text.asp?2012/11/1/13/100404
Introduction
Meju is the principal ingredient and the starter culture of Korean traditional fermented soybean foods such as doenjang and kanjang, and these fermented soybean products are well-known for their various health benefits, which include anticancer and antimutagenic effects. [1],[2] They have been traditionally manufactured in the home for a long time. Although meju manufactured via the traditional method has good taste and adds functionalities to fermented soybean foods, problems regarding its safety have been consistently reported, because a number of naturally occurring micro-organisms are related to traditional meju fermentation. Therefore, attempts have been made to develop meju manufacturing methods that take advantage of the traditional method and increase food safety, [3],[4],[5],[6] and one of these methods involves the use of effective and beneficial micro-organisms as starter cultures.
There are two basic types of Korean meju: rectangular-type block meju which is fermented naturally, and grain-type meju, which is usually inoculated commercially with Aspergillus oryzae (koji). [7] The micro-organisms found most frequently in traditional or commercial meju are Bacillus strains (10 6 -10 8 cfu/g), lactic acid bacteria (10 5 -10 8 cfu/g), and molds (10 6 -10 8 cfu/g), particularly Aspergillus oryzae.[8]
Colorectal cancer (CRC) is one of the major causes of cancer-related mortality in both men and women in most of the developed world. [9] The risk factors of CRC include age, family history, inflammatory bowel disease (IBD), and environmental and dietary procarcinogens. [10] The association between colitis and colorectal cancer is currently broadly accepted, and chronic inflammation is assumed to be a direct cause of colitis-associated cancer. [11]
Cycloooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) are the primary enzymes related to colon carcinogenesis in the context of chronic inflammation. COX-2 is expressed by reactive oxygen species and tumor promoters, as well as the stimulation of proinflammatory cytokines such as TNF-α. Additionally, upregulated COX-2 generates prostaglandins that can promote cell growth and angiogenesis. iNOS produces nitric oxide (NO) which stimulates COX-2 activity and increases the inactivation of the p53 tumor suppressor gene. [12],[13] Moreover, the lack of p53 activation results in the down regulation of cyclin-dependent kinase (Cdk) inhibitors such as p21 and proapoptotic genes such as Bax. [14]
In the previous study, we isolated major probiotic micro-organisms from a variety of meju samples, and determined that Bacillus subtilis-SKm and Lactococcus lactis-GAm evidenced high enzyme activities and significant probiotic effects. [15] Therefore, we manufactured grain-type meju using the probiotic mixed starter cultures–Aspergillus oryzae, Bacillus subtilis-SKm and Lactococcus lactis-GAm–and assessed their inhibitory effects on colon carcinogenesis using an AOM and DSS-induced colitis-associated colon carcinogenesis mice model. We observed the general conditions of the mice and evaluated the serum levels of proinflammatory cytokines such as IL-6 and TNF-α. Histological analysis was also conducted via hematoxylin and eosin (H and E) staining, and inflammation- and apoptosis-related gene levels were also analyzed in the colonic tissues of mice.
Materials and Methods
Preparation of starter cultures
First, we isolated and identified several micro-organisms from traditional meju. Among them, we selected Bacillus subtilis-SKm (KFCC11520P) and Lactococcus lactis-GAm (KFCC11510P) which evidenced excellent enzyme activities and probiotic effects for the preparation of mixed starter cultures. [15] Bacillus subtilis-SKm and Lactococcus lactis-GAm were inoculated with nutrient broth and MRS broth, respectively, and incubated for 24-48 hours at 37°C before use. Aspergillus oryzae koji was purchased from Chungmoo Fermentation (Ulsan, Korea) and maintained at -20°C prior to use.
Preparation of grain-type meju
The grain-type meju using mixed starter cultures was prepared via the following method, and was designated as Aspergillus oryzae, Bacillus subtilis-SKm, and Lactococcus lactis-GAm inoculated meju (ABL-m). For comparison with this meju in further studies, commercial grain-type meju (CG-m) purchased from Alalee Food Co. (Koryung, Korea) was used.
For the preparation of the meju, soybeans were washed and soaked in water at 20°C for 12 hours, then steamed at 121°C for 30 minutes in an autoclave. The steamed soybeans were then cooled down to 45-50°C, and inoculated with 0.2% (10 6 spores/g) Aspergillus oryzae and 10 6 cfu/g Bacillus subtilis-SKm prior to incubation at 30°C for 48 hours. The fermented soybeans were then inoculated with 10 6 cfu/g of Lactococcus lactis-GAm and incubated for 24 hours at 37°C.
The meju could be fermented at 37°C for more than 6 weeks to generate the final products of doenjang (soypaste) and kanjang (soysauce). The method for the preparation of meju, doenjang, and kanjang is schematized in [Figure 1].
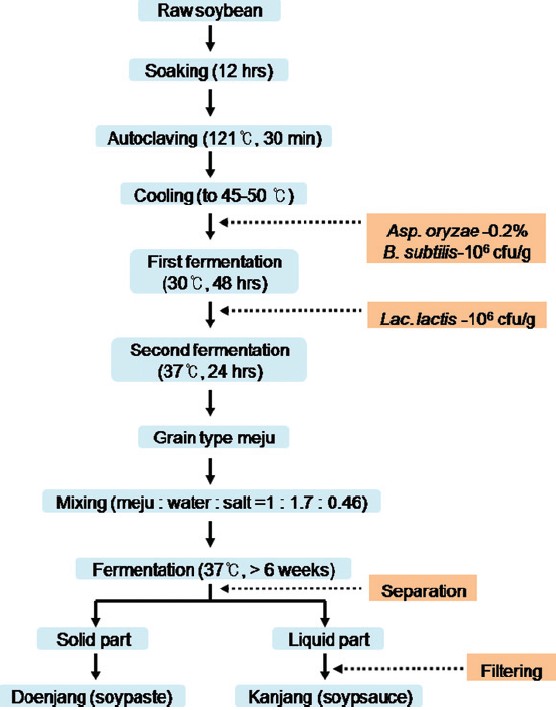 |
Figure 1: Preparation of the grain-type meju and meju-derived fermented soybean foods, doenjang and kanjang[7] Click here to view |
Azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced colorectal carcinogenesis model
Specific pathogen-free, 7-week-old C57BL/6 mice (Central Lab animal Inc., Seoul, Korea) were randomly allocated into 8-mouse groups, and housed under standard conditions (50-60% humidity, 12 hours dark/12 hours light cycle). Food and water was supplied ad libitum.
Mice in the sample treatment groups were administered single intraperitoneal injections of azoxymethane (10 mg/kg, AOM, Sigma Co., St. Louis, MO, USA). Beginning 2 and 5 weeks after the injections, the animals received 2.5% dextran sulfate sodium (DSS, Mw 30,000-50,000, MP Bio, Solon, OH, USA) in their drinking water for 7 days. Meju (ABL-m and CG-m) were orally administered 2 weeks after the injection for 4 weeks. The normal group received 0.1 ml PBS as a vehicle after the onset of the experiment for 4 weeks without AOM and DSS, and the control group was administered a single intraperitoneal injection of AOM followed by 2.5% DSS in the drinking water after 2 and 5 weeks for 7 days. PBS was orally administered 2 weeks after the injection for a period of 4 weeks. The total experimental period was 8 weeks. Experiments were conducted in accordance with protocols approved by the Animal Ethics Committee of Pusan National University.
Serum and tissue sample preparation
At the end of the experiment, blood samples were collected from each animal without anticoagulant treatment. Serum was separated from the blood via 10 minutes of centrifugation at 3,500 rpm. Colon tissues were collected directly from the mice, the length and weight of the tissues were measured, and the samples were frozen in liquid nitrogen. The samples were stored at -80°C until use.
Histological examination
At the end of the experimental period, the colon tissues were removed and cleaned in saline to remove fecal residue. The colon tissues were fixed in 10% (v/v) buffered formalin and embedded in paraffin. Four μm-thick slices from paraffin sections were stained with hematoxylin and eosin (H and E) prior to microscopic observation. Histological analysis was conducted by a pathologist who was unaware of the experimental protocol. [16]
Proinflammatory cytokine analysis in serum
Concentrations of the proinflammatory cytokines IL-6, IL-12, IL-17, TNF-α, and IFN-γ were determined using a commercial ELISA kit (ELISA MAX, Biolegend, San Diego, CA, USA).
Reverse transcription polymerase chain reaction
Gene expression was measured via reverse transcription polymerase chain reaction (RT-PCR) in an ExiCycler (Bioneer, Daejeon, Korea). In brief, total RNA was isolated from tissues in mice using Trizol reagent (Invitrogen, Carlsbad, CA, USA). One microgram of total RNA was used for first-strand cDNA synthesis using Superscript II reverse transcriptase (BD Bioscience, Palo Alto, CA, USA). Reverse transcription was conducted at 30°C for 10 minutes, 42°C for 30 minutes, and 99°C for 5 minutes to inactivate the avian myeloblastosis virus RTXL. Amplification was conducted in a master-cycler (Eppendorf, Hamburg, Germany) with denaturation at 94°C for 1 minute, annealing at 54°C for 1 minute, extension at 72°C for 30 seconds for 25 cycles, and a final extension at 72°C for 7 minutes. The amplified PCR products were then run on 1.0% agarose gel and stained with ethidium bromide (EtBr), and visualized under UV light. The intensities of the bands were estimated via densitometry (Multi Gauge V3.0 software, Fujifilm Life Science, Tokyo, Japan).
Statistical analysis
All results obtained were analyzed in time design using the general linear model procedure of the SAS System (SAS Institute, Cary, NC, USA). Data are expressed as means ± standard error values. Means with different letters differ significantly (P < 0.05) according to Duncan’s multiple range tests.
Results
General observations
The body weights of all groups increased during the experimental period. The rate of increase differed somewhat among the groups; however, no considerable differences in the final body weight were noted [Table 1]. Unlike the DSS-induced colitis mice model, [15] no significant losses in body weight were noted in the AOM and DSS-induced colitis-associated cancer mice models during the experimental period. Liver and spleen weights were elevated significantly in the control group relative to the normal group and diminished slightly in the meju treatment group [Table 2]. In particular, the spleen weights of the ABL-m-treated group were significantly lighter than those of the control group. The colon lengths of each group did not differ considerably; however, the colon weights in the control group were increased significantly compared with the normal group [Table 3]. The colon weight and weight/length ratio were reduced slightly in the meju treatment group.
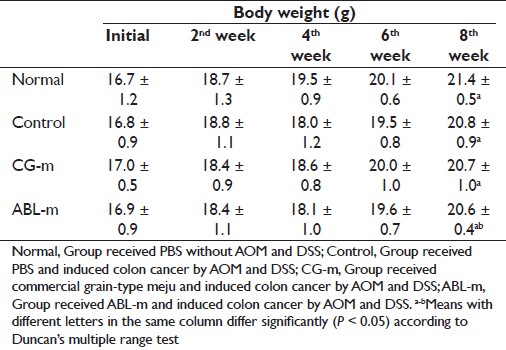 |
Table 1: Changes in the body weight of AOM and DSS-induced colon cancer mice during meju treatment Click here to view |
 |
Table 2: Effects of meju treatment on the organ weights of AOM and DSS-induced colon cancer mice at the end of the experiment Click here to view |
 |
Table 3: Effects of meju treatment on the colon lengths and colon weights of AOM and DSS-induced colon cancer mice at the end of the experiment Click here to view |
Histological observations
Mice in the normal group did not evidence colonic inflammation, injury, or neoplasms [Figure 2]a. Nodular or polypoid colonic tumors were observed macroscopically in the colons of the mice treated with AOM and DSS. The colonic tissues of mice receiving AOM and DSS evidenced mild to severe inflammation, characterized by crypt damage and inflammatory cell infiltration. The aforementioned mucosal thickening in the mice receiving AOM and DSS, as mentioned above, appeared to be attributable to the burden of colonic neoplasms. These phenomena did not differ distinctly between the meju-fed groups [Figure 2]b-d. However, colonic neoplasms were analyzed and diagonized as shown in [Figure 3]. AOM and DSS treatment resulted in a 100% incidence of colonic neoplasms, which were most frequently observed in the middle and distal colon. The control group evidenced a 100% incidence of colon neoplasms, with a multiplicity of 14.5. The administration of CG-m and ABL-m reduced the total multiplicity of colon neoplasms by 20% and 27%, respectively. More noteworthy was that ABL-m significantly retarded the development of large neoplasms (diameter > 1 mm) by more than 40%.
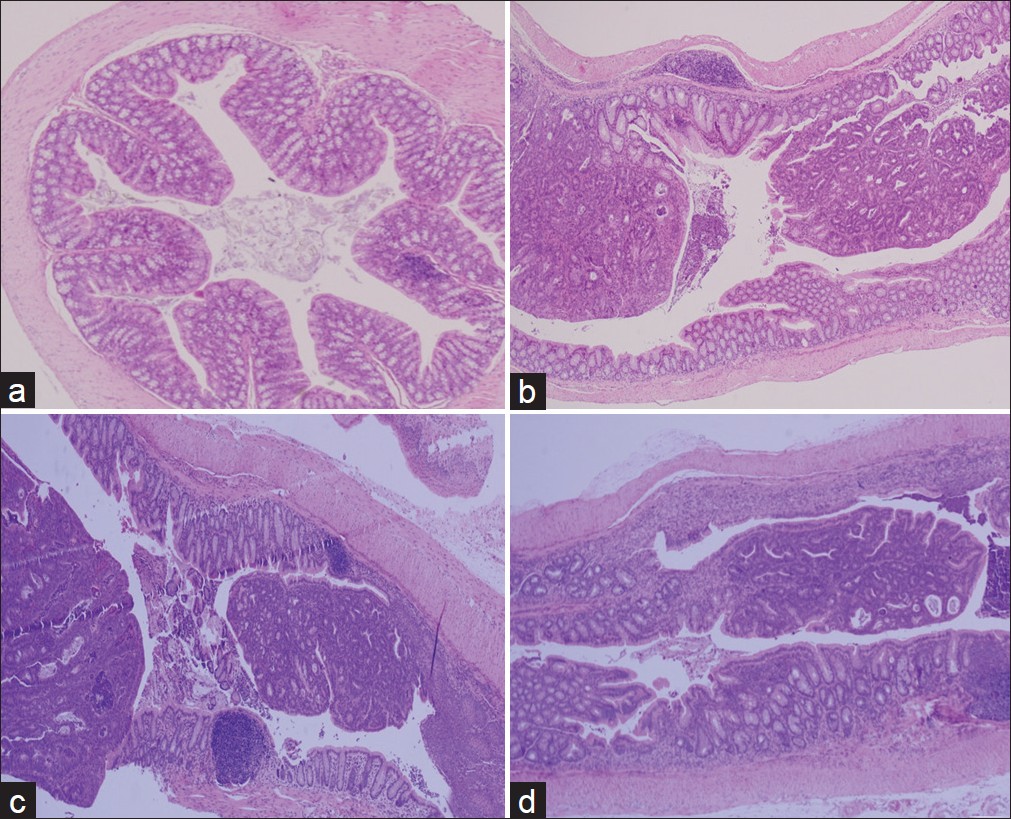 |
Figure 2: Representative histological images of colonic mucosa of mice in the normal group (a), control group (b), and meju treatment groups (c,d) (×200 magnification). Normal, Group received PBS without AOM and DSS; Control, Group received PBS and induced colon cancer by AOM and DSS; CG-m, Group received commercial grain-type meju and induced colon cancer by AOM and DSS; ABL-m, Group received ABL-m and induced colon cancer by AOM and DSS Click here to view |
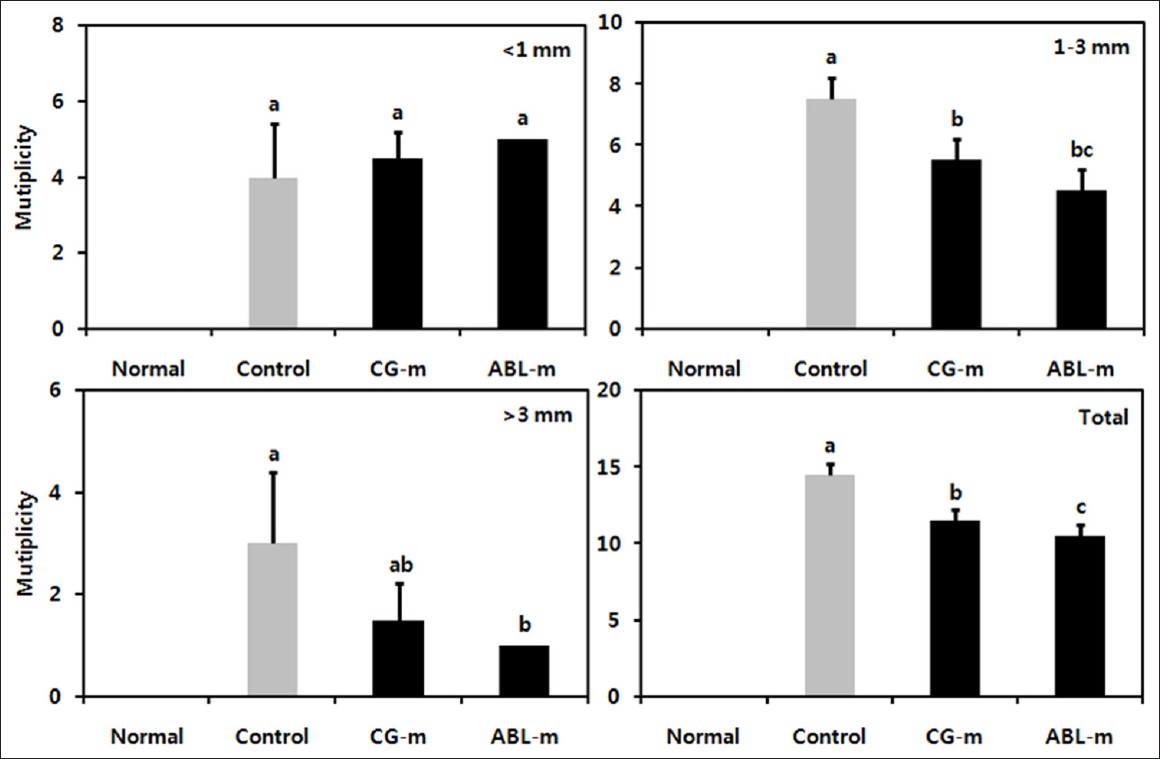 |
Figure 3: Effects of meju treatment on the incidence and size of colonic neoplasms in mice with colon cancer induced by AOM and DSS. Normal, Group received PBS without AOM and DSS; Control, Group received PBS and induced colon cancer by AOM and DSS; CG-m, Group received commercial grain-type meju and induced colon cancer by AOM and DSS; ABL-m, Group received ABL-m and induced colon cancer by AOM and DSS. (a-c) Means with different letters differ significantly (P < 0.05) according to Duncan’s multiple range test Click here to view |
Proinflammatory cytokine levels
Many previous studies have also demonstrated a profound correlation between colitis-associated cancer and the production of IL-6, IL-12, TNF-α, and IFN-γ. [17],[18] Therefore, the effect of the meju on systemic cytokine secretion in colitis-associated cancer was assessed herein. IL-6, IL-12, TNF-α, and IFN-γ levels were increased significantly in mice receiving AOM and DSS versus the mice in the normal group. However, IL-6, IL-12, TNF-α, and IFN-γ levels were reduced significantly in the meju-fed animals [Figure 4].
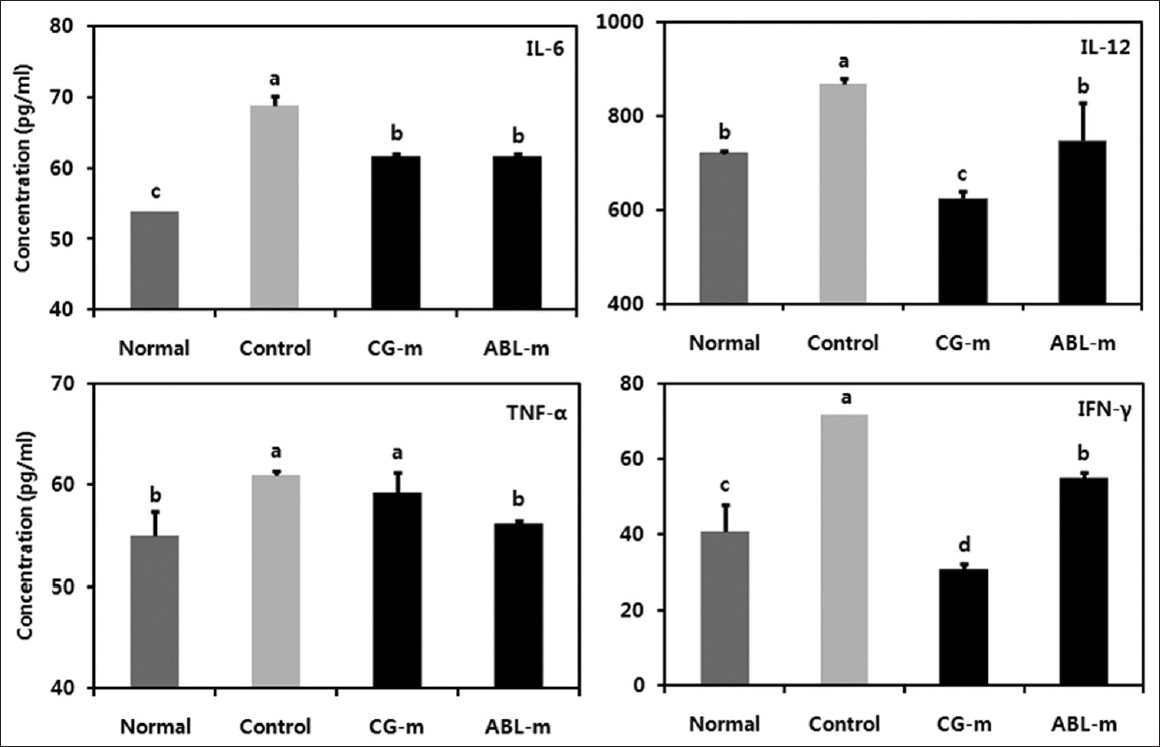 |
Figure 4: Effects of meju treatment on serum proinflammatory cytokines in mice with colon cancer induced by AOM and DSS. Normal, Group received PBS without AOM and DSS; Control, Group received PBS and induced colon cancer by AOM and DSS; CG-m, Group received commercial grain-type meju and induced colon cancer by AOM and DSS; ABL-m, Group received ABL-m and induced colon cancer by AOM and DSS. (a-d) Means with different letters differ significantly (P < 0.05) according to Duncan’s multiple range test Click here to view |
iNOS and COX-2 expression in colon tissue
It is now broadly accepted that chronic inflammation contributes substantially to colitis-associated colon carcinogenesis. Therefore, it was believed that the preventive activity of the meju was mediated via its anti-inflammatory properties. iNOS expression is a reliable indicator of mucosal inflammation, and iNOS is responsible for the production of large quantities of nitric oxide, which can cause DNA-damage and simultaneously inhibit DNA repair mechanisms. In this regard, the expression of iNOS in colonic tissue was dramatically upregulated by AOM and DSS treatment, and significantly down-regulated by the administration of meju, particularly ABL-m [Figure 5].
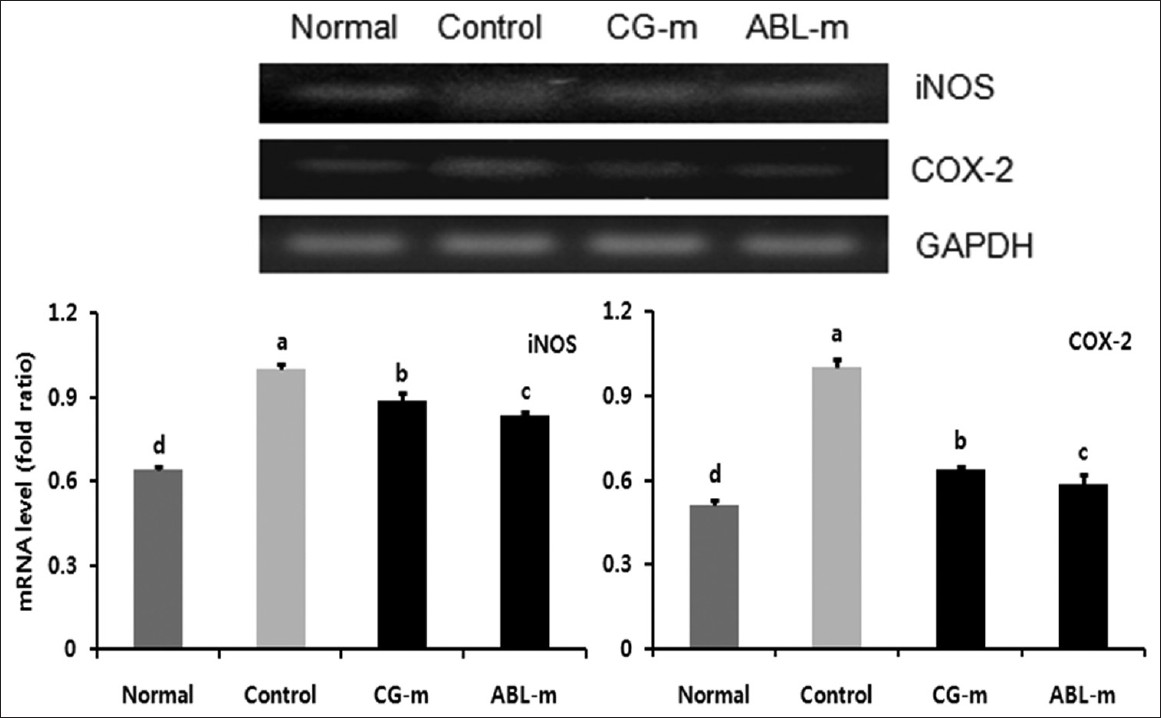 |
Figure 5: Effects of meju treatment on mRNA expressions of iNOS and COX-2 in the colon tissues of mice with colon cancer induced by AOM and DSS. Normal, Group received PBS without AOM and DSS; Control, Group received PBS and induced colon cancer by AOM and DSS; CG-m, Group received commercial grain-type meju and induced colon cancer by AOM and DSS; ABL-m, Group received ABL-m and induced colon cancer by AOM and DSS. (a-d) Means with different letters differ significantly (P < 0.05) according to Duncan’s multiple range test Click here to view |
Expression of bax, Bcl-2, p21, and p53 in Colon Tissue
To assess the protective mechanisms relevant to colon carcinogenesis induced by meju treatment, the mRNA expressions of the pro-apoptotic protein, Bax, and the anti-apoptotic protein, Bcl-2, were measured in colon tissue. As shown in [Figure 6], pro-apoptotic Bax levels were reduced, and levels of anti-apoptotic Bcl-2 increased in the control group. Additionally, significant changes were observed in the meju treatment group, especially the ABL-m-treated animals, relative to the control group. Accordingly, these results indicated that the inhibitory effects of meju administration on colitis-associated cancer were induced by the apoptosis of colon cancer cells via the Bax and Bcl-2 dependent pathways.
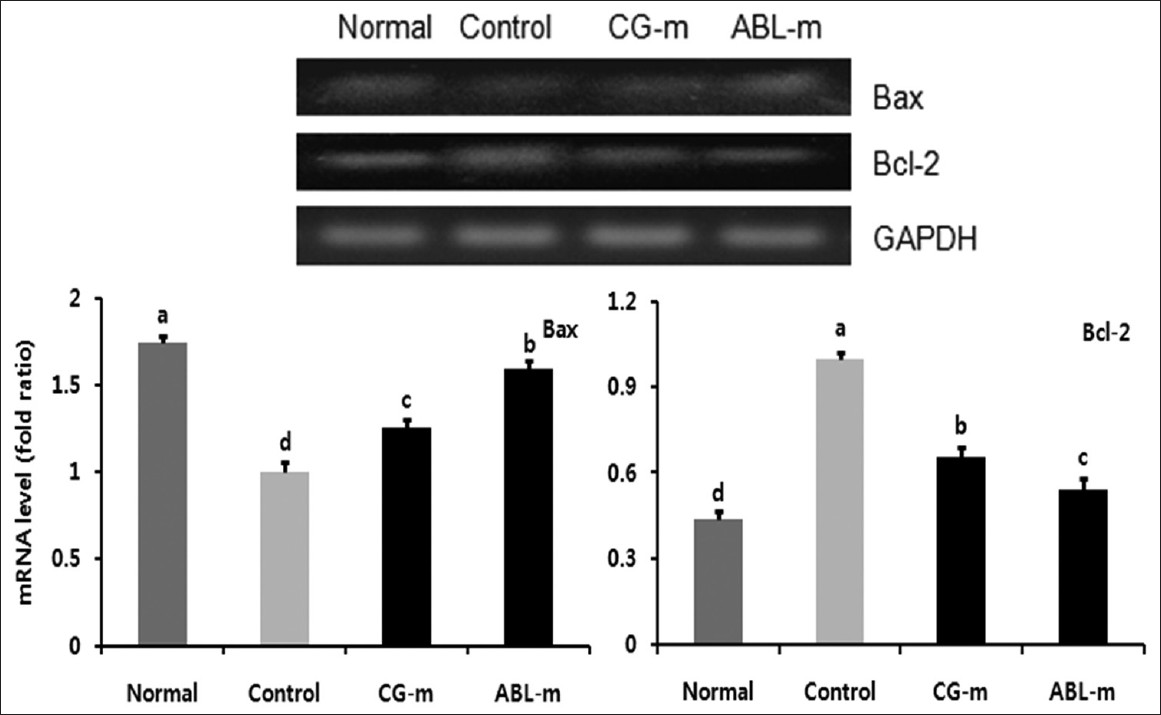 |
Figure 6: Effects of meju treatment on mRNA expressions of Bax and Bcl-2 in the colon tissue of mice with colon cancer induced by AOM and DSS. Normal, Group received PBS without AOM and DSS; Control, Group received PBS and induced colon cancer by AOM and DSS; CG-m, Group received commercial grain-type meju and induced colon cancer by AOM and DSS; ABL-m, Group received ABL-m and induced colon cancer by AOM and DSS. (a-d) Means with different letters differ significantly (P < 0.05) according to Duncan’s multiple range test Click here to view |
p53, a tumor suppressor gene, is a key biosensor of inflammatory stress; p53 is activated by phosphorylation at Ser 15 during inflammatory stress and is inactivated by chronic inflammatory stimuli. [19] Moreover, when the p53 gene is inactivated, the p21 gene, a Cdk inhibitor, also undergoes a down regulation. Expression levels of p21 and p53 were significantly reduced in the mice in which colorectal cancer was induced by AOM and DSS; however, the expressions of p21 and p53 were increased by meju treatment [Figure 7].
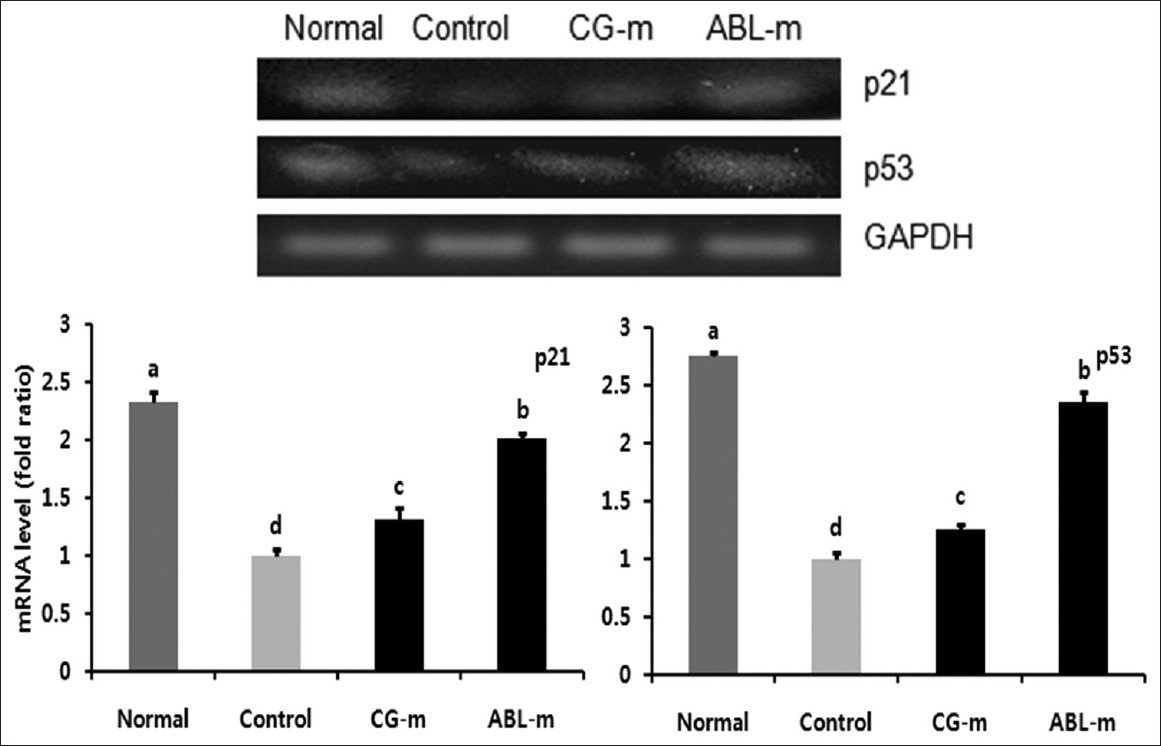 |
Figure 7: Effects of meju treatment on mRNA expressions of p21 and p53 in the colon tissues of mice with colon cancer induced by AOM and DSS. Normal, Group received PBS without AOM and DSS; Control, Group received PBS and induced colon cancer by AOM and DSS; CG-m, Group received commercial grain-type meju and induced colon cancer by AOM and DSS; ABL-m, Group received ABL-m and induced colon cancer by AOM and DSS. (a-d) Means with different letters differ significantly (P < 0.05) according to Duncan’s multiple range test Click here to view |
Discussion
As mentioned above, it is well-known that doenjang and kanjang, the end products of meju, exert anticancer effects. The principal objective of this study was to evaluate the potential inhibitory effects of meju fermented by mixed probiotic micro-organisms in an AOM and DSS model of colon carcinogenesis. Additionally, meju, which has a strong inhibitory effect on cancer, is expected to produce doenjang and kangjang with excellent quality and functionality. The results of this study indicated that meju produced using a probiotic mixed starter culture exerts an inhibitory effect against colon carcinogenesis in this model.
The increase in the ratio of colon weight to colon length was a consequence of apparent mucosal thickening and the formation of neoplasia. [20] This symptom was noted in the AOM and DSS-treated control group and alleviated in mice treated with meju, particularly ABL-m.
The pathogenesis of colitis-associated cancer is generally known to occur via a stepwise progression from inflamed and hyperplastic cells, through flat dysplasia, to adenocarcinoma. [21] However, mucosal inflammation may result in colonic carcinogenesis via several mechanisms, including increased crypt cell proliferation, changes in crypt cell metabolism, and alteration in colonic microbiota. [22] In the colon, the number of epithelial cells in the crypts is strictly regulated by a balance between cell proliferation and cell death that maintains homeostasis. In neoplastic tissues, changes in cell proliferation and apoptosis are regarded as a common point in the pathogenesis of tumor formation. [23] In this study, the effects of meju on cellular proliferation and apoptosis may contribute to their lowering activity in the multiplicity of colonic neoplasia.
Cytokines are recognized to perform a major role in the immunopathogenesis of inflammatory bowel disease and the promotion of neoplastic transformation. [24] They are encoded by the target genes of the NF-κB-activation pathway, which are associated with tumor development and progression. [25],[26] The blockage of proinflammatory cytokines such as TNF-α has been shown to be highly effective in the treatment of colorectal carcinogenesis associated with chronic colitis. [27] In this study, mice fed with the meju produced using mixed starter cultures evidenced significantly lower inductions of IL-6, IL-12, TNF-α, and IFN-γ relative to the control group. These results demonstrate that the meju appears to exert anti-inflammatory and growth inhibitory effects on colorectal cancer development via the regulation of these cytokines.
In this study, the levels of iNOS and COX-2 in mice with colon cancer increased significantly; this finding was consistent with many previous studies. [20],[28] This evidence suggests that the process of colitis-associated colon carcinogenesis may be accelerated via nitric oxide synthesis. It has been theorized that nitric oxide production may function as one of the reactive nitrogen species that have been implicated in chronic inflammation-related colon cancer. [29] Intestinal COX-2 levels manifested the same tendency as iNOS level. COX-2 is involved in a variety of pathological processes and regulates the production of prostaglandins, which can promote cell growth, angiogenesis, and immunosuppression. [30] In chronic inflammation, nitric oxide stimulates COX-2 activity and reduces p53 expression, thereby contributing to clone cellular expansion and genomic instability. [31] The mechanism by which meju prevents colitis-associated colon carcinogenesis may specifically involve iNOS and COX-2.
In the initial stages of colon carcinogenesis, the activation of proto-oncogenes and the inactivation of tumor suppressor genes followed by increased cancer cell proliferation or the evasion of apoptosis represents a common pathway at the cellular level. In this study, pro-apoptotic Bax levels decreased and anti-apoptotic Bcl-2 increased in the control group; additionally, significant changes were noted in the ABL-m treatment group relative to the control group [Figure 5]. These results suggested that the inhibitory effects of meju administration on colitis-associated cancer involved the apoptosis of colon cancer cells via a Bax- and Bcl-2-dependent pathway. Additionally, p21 and p53 expression levels were reduced significantly in colorectal cancer-induced mice, and were increased in the meju treatment group [Figure 7]. These results indicate that p53 is a target of meju (ABL-m)-induced apoptosis or cancer cell senescence, and meju also induces cell cycle arrest in cancer cells, possibly via the promotion of p53 binding to the cell cycle inhibitor p21. [32],[33]
In our other study, doenjang prepared with ABL-m was also shown to exert an anticancer effect in vitro and an inhibitory effect against AOM and DSS-induced colon carcinogenesis in vivo, and these effects were more profound than those of commercial meju. [14] Therefore, meju produced using probiotic mixed starter culture appears to be an effective method to improve the functionality of meju and doenjang.
Conclusions
Grain-type meju fermented by probiotic mixed starter cultures was shown to exert inhibitory effects on the progression of colitis-associated colon carcinogenesis in AOM and DSS animal model. These beneficial effects were correlated with an amelioration of colon cancer symptoms such as the reduction of colon weight, reduced numbers of neoplasia, and reduced serum proinflammatory cytokine levels of IL-6, IL-12, TNF-α, and IFN-g. Our data also indicated that the inhibitory effects of meju on colon carcinogenesis are explained by down regulation of iNOS and COX-2, promotion of cancer cell apoptosis by regulating Bax and Bcl-2, and upregulations of p21 and p53 in the colonic tissues.
References
| 1. | Jung KO, Park SY, Park KY. Longer aging time increases the anticancer and antimetastatic properties of deonjang. Nutrition 2006;22:539-45.  [PUBMED] |
| 2. | Park KY, Jung KO, Rhee SH, Choi YH. Antimutagenic effects of doenjang (Korean fermented soypaste) and its active compounds. Mutat Res 2003;523-524:43-53.  [PUBMED] |
| 3. | Im MH, Choi JD, Chung HC, Lee SH, Lee CW, Choi C, et al. Improvement of meju preparation method for the production of Korean traditional kanjang (say sauce). Korean J Food Sci Technol 1998;30:608-14.  |
| 4. | Kim YS, Park CW, Kim SJ, Park SJ, Ryu CH, Cho HJ, et al. Preparation of mushroom mycelia-cultured traditional meju with enhanced anticarcinogenecity and sensory quality. J Korean Soc Food Sci Nutr 2002;31:986-93.  |
| 5. | Kim DH, Lee KH, Yook HS, Kim JH, Shin MG, Byun MW. Quality characteristics of gamma irradiated grain shape improved meju. Korean J Food Sci Technol 2000;32:640-45.  |
| 6. | Ko BK, Kim KM, Hong YS, Lee CH. Metabolic assessment of fermentative capability of soybean starter treated with high pressure. J Agric Food Chem 2010;58:8738-47.  [PUBMED] |
| 7. | Park KY, Jung KO. Fermented soybean products as functional foods: Functional properties of doenjang (fermented soybean paste). In: John S, editor. Asian functional foods. FL, USA: CRC; 2005. p. 555-96.  |
| 8. | Choi KS, Lee HJ, Kwon DJ. Physicochemical and microbiological properties of Korean traditional Meju. Korean J Food Preserv 2009;16:217-22.  |
| 9. | Janakiram NB, Rao CV. Molecular markers and targets for colorectal cancer prevention. Acta Pharmacol Sol 2008;29:1-20.  [PUBMED] |
| 10. | Benson AB 3rd. Epidemiology, disease progression, and economic burden of colorectal cancer. J Manag Care Pharm 2007;13:5-18.  |
| 11. | Manasa K, Mari MK, Emiko M. Animal models of colitis-associated carcinogenesis. J Biomed Biotechnol 2011; Article ID 342637.  |
| 12. | Muller-Decker K, Furstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog 2007;46:705-10.  |
| 13. | Kawanishi S, Hikaru Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory disease in relation to inflammation-related carcinogenesis. Bio Chem 2006;387:365-72.  |
| 14. | Danuta MG, Natalia T. Molecular mechanisms of carcinogenesis. In: Wanda BD, editor. Carcinogenesis and anticarcinogenic food components. FL, USA: CRC; 2006. p. 13-36.  |
| 15. | Jeong JK. Improvement of quality and probiotic effect of meju and doenjang prepared with mixed starter cultures Doctoral Thesis . Busan, Korea: Pusan National University; 2012. p. 111-125  |
| 16. | Melgar S, Karlsson L, Rehnstrom E, Karlsson A, Utkovic H, Jansson L, et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int immunopharmacol 2008;8:836-44.  |
| 17. | Villegas I, Sánchez-Fidalgo S, de la Lastra CA Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Mol Nutr Food Res 2011;55:259-67.  |
| 18. | Tsvetkov P, Asher G, Reiss V, Shaul Y, Sachs L, Lotem J. Inhibition of NAD(P) H: Quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proc Natl Acad Sci USA 2005;102:5535-40.  [PUBMED] |
| 19. | Hofseth LJ, Saito S, Hussain SP, Espey MG, Miranda KM, Araki Y, et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci USA 2003;100:14-8.  [PUBMED] |
| 20. | Li H, Wu KK, Li ZJ, Chan KM, Wong CM, Ye CG, et al. 2,3′,4,4′,5′-pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits colitis-associated colorectal carcinogenesis in mice. Br J Pharmacol 2010;160:1352-61.  |
| 21. | Tanaka T, Kohno H, Murakami M, Shimada R, Kagami S. Colitis-related rat colon carcinogenesis induced by 1-hydroxyanthraquinone and methyazoxymethanol acetate. Oncol Rep 2000;7:501-8.  |
| 22. | Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in human and animal models. Carcinogenesis 2003;24:353-62.  [PUBMED] |
| 23. | Kohno H, Suzuki R, Curini M, Epifano F, Maltese F, Gonzales SP, et al. Dietary administration with prenyloxycoumarins, auraptene and collinin, inhibits colitis-related colon carcinogenesis in mice. Int J Cancer 2006;118:2936-42.  [PUBMED] |
| 24. | Fantini MC, Pallone F. Cytokines: From gut inflammation to colorectal cancer. Curr Drug Targets 2008;9:375-80.  [PUBMED] |
| 25. | Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci 2003;94:965-73.  |
| 26. | Tanaka K. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog 2009;8:5.  [PUBMED] |
| 27. | Popivaniva BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 2008;118:560-70.  |
| 28. | Suh N, Paul S, Hao X, Simi B, Xiao H, Rimando AM, et al. Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats. Clin Cancer Res 2007;13:350-5.  |
| 29. | McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cell during inflammatory bowel disease. J Clin Invest 1996;98:136-41.  |
| 30. | Kim SJ, Kim KW, Kim DS, Kim MC, Jeon YD, Kim SG, et al. The protective effect of Cassia obtusifolia on DSS-induced colitis. Am J Chin Med 2011;39:565-77.  |
| 31. | Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis, linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol 2001;281:G626-34.  |
| 32. | Lin HY, Sun M, Tang HY, Simone TM, Wu YH, Grandis JR, et al. Resveratrol causes COX-2- and p53-dependent apoptosis in head and neck squamous cell cancer cells. J Cell Biochem 2008;104:2131-42.  |
| 33. | Liontas A, Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res 2004;24:987-98.  |
Authors
Dr. Ji-Kang Jeong, Department of Food Science and Nutrition, Pusan National University, Busan, Korea
Dr. Hee-Kyung Chang, Department of Pathology, Kosin University Gospel Hospital, Busan, Korea
Dr. Kun-Young Park, Department of Food Science and Nutrition, Pusan National University, Busan, Korea
Figures
[Figure 1], [Figure 2], [Figure 3], [Figure 4], [Figure 5], [Figure 6], [Figure 7]
Tables
