Sneha Sundaram1, Amy R Johnson1, Liza Makowski2
1 Department of Nutrition, Nutrition Obesity Research Center, and Lineberger Comprehensive Cancer Center, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, 135 Dauer Drive, CB #7461, Chapel Hill, NC, 27599, USA
2 Department of Nutrition and Medicine, Nutrition Obesity Research Center, and Lineberger Comprehensive Cancer Center, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, 135 Dauer Drive, CB #7461, Chapel Hill, NC, 27599, USA
| Date of Submission | 21-Mar-2013 |
| Date of Acceptance | 06-Aug-2013 |
| Date of Web Publication | 09-Oct-2013 |
Correspondence Address:
Liza Makowski
Department of Nutrition and Medicine, Nutrition Obesity Research Center, and Lineberger Comprehensive Cancer Center, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, 135 Dauer Drive, CB #7461, Chapel Hill, NC, 27599
USA
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/1477-3163.119606
Abstract
Historically, cancer research has focused on identifying mutations or amplification of genes within the tumor, which informed the development of targeted therapies against affected pathways. This work often considers tumor cells in isolation; however, it is becoming increasingly apparent that the microenvironment surrounding tumor cells strongly influences tumor onset and progression. This is the so-called “seed and soil” hypothesis wherein the seed (cancer cell) is fed and molded by the metabolites, growth factors, modifications of the extracellular matrix or angiogenic factors provided by the soil (or stroma). Currently, 65% of the US population is obese or overweight; similarly staggering figures are reported in US children and globally. Obesity mediates and can exacerbate, both normal and tumor microenvironment dysfunction. Many obesity-associated endocrine, metabolic and inflammatory mediators are suspected to play a role in oncogenesis by modifying systemic nutrient metabolism and the nutrient substrates available locally in the stroma. It is vitally important to understand the biological processes linking obesity and cancer to develop better intervention strategies aimed at curbing the carcinogenic events associated with obesity. In this review, obesity-driven changes in both the normal and tumor microenvironment, alterations in metabolism, and release of signaling molecules such as endocrine, growth, and inflammatory mediators will be highlighted. In addition, we will discuss the effects of the timing of obesity onset or particular “windows of susceptibility,” with a focus on breast cancer etiology.
Keywords: Basal-like breast cancer, health disparities, inflammation, macrophage, tumor subtype, window of susceptibility
How to cite this article:
Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. J Carcinog 2013;12:19
How to cite this URL:
Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. J Carcinog [serial online] 2013 [cited 2021 Oct 13];12:19. Available from: https://carcinogenesis.com/text.asp?2013/12/1/19/119606
Introduction
Cancer is the second leading cause of death in the developed world, surpassed only by heart disease [1] and obesity is increasingly recognized as an oncogenic factor. [2] The World Health Organization estimates that 500 million adults and almost 43 million children under the age of five are obese worldwide. [3] In the US, the incidence of obesity may be plateauing, but the prevalence of obesity (body mass index [BMI] >30) remains at 30%. Individuals who are overweight (BMI > 25 and < 30) represent a staggering 65% of the population. [4] Childhood obesity is of particular concern [5] as healthcare professionals are increasingly treating children for chronic diseases and endocrine disorders such as early menarche that are linked to cancer predisposition. [6],[7] In 2003, the results of large US and UK cohort studies first reported the striking association between obesity and cancer. [2],[8],[9],[10] Since then, it is now estimated that being overweight or obese contributes to 20% of US cancer deaths by influencing cancer onset. [11] For a review of common cancers associated with an increased BMI refer to. [12]
This review will discuss obesity-mediated mechanisms leading to tumor progression. Specific foods or nutrients such as saturated fats, processed foods, or charred meat that can act as carcinogens have been reviewed elsewhere and are beyond the scope of this review. [7],[13],[14],[15],[16],[17] Metabolic alterations associated with obesity have been reviewed in detail by Johnson et al. [18] and will be briefly discussed herein. It is now widely accepted that obesity may promote cancer through several mechanisms and the effects of obesity on cancer risk is the primary topic covered in this review, with a focus on a specific breast cancer (BC) subtype called basal-like breast cancer (BBC).
Obesity, Breast Cancer and Windows of Susceptibility
BC represents the highest incidence of cancers affecting women. It is the second most fatal cancer type. [19],[20] It is likely that it is likely that throughout the lifespan particular “windows of susceptibility” exist during which during which obesity plays a disproportionately greater role in promoting BC onset. Obesity, which disturbs tissue homeostasis, is one of the few modifiable BC risk factors; therefore, in order to develop more effective therapeutic strategies aimed at combating obesity-associated BC, it is critical to first understand the molecular mechanisms orchestrating the effects of obesity on BC. Our work has focused on specific periods of heightened susceptibility to BC due to the presence of an obese environment, such as the post-partum period. [21] In addition, we have studied the normal breast microenvironment in both preclinical and human models to understand the role that obesity plays in cell-cell crosstalk and to delineate the underlying factors contributing to increased BC risk. [21],[22],[23]
With the advent of high-throughput gene sequencing and expression analysis and the construction of The Cancer Genome Atlas, the classification of tumor subtypes with defined risks and clinical outcomes has opened the door to consider subtype-specific mechanisms. [24],[25] Considering that BC is a heterogeneous disease with many identified intrinsic subtypes (including luminal A and B, basal-like, claudin-low, and other subtypes), [26] it is not surprising that intricate relationships exist between modifiable and non-modifiable risk factors in each subtype. Considering BCs overall, the relationship between obesity and risk is complex having no (or even protective) effects on premenopausal BC risk, yet obesity is associated with an increased risk of postmenopausal BC. [27] With the ability to stratify BCs according to subtype, epidemiologic studies have demonstrated that obesity is strongly associated with an increased risk of BBC in both pre- and postmenopausal women, [27] whereas luminal BCs are associated with obesity solely during the postmenopausal period. BBCs are aggressive cancers, typically estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 negative (so-called “triple-negative BCs”) and as such, targeted therapies for BBCs are currently unavailable. [28] These tumors are highly proliferative, patients have poor overall survival and are diagnosed predominantly in young African-American women, particularly obese women. [4],[26],[27] Millikan et al. estimated that up to 68% of BBCs could be prevented by encouraging more women to breastfeed and by reducing obesity [26],[27] suggesting that this aggressive subtype of BC may be preventable through lifestyle modifications. Determining mechanistic risk factors could help address the need to reduce BBC risk as well as observed health disparities. Studies in preclinical models of other BC subtypes demonstrate that diet-induced obesity is associated with shortened mammary tumor latency [29],[30] and our findings demonstrate that this is also true for obesity-linked BBC. [21]
Mechanisms of obesity related cancer
Growth factors and hormones
The mechanism(s) by which obesity induces carcinogenesis is likely to vary by cancer site, although several obesity-related systemic alterations may contribute to cancer onset globally through nutrient sensitive signaling cascades, such as the insulin/insulin-like growth factor (IGF-1) and PI3K/Akt/mammalian target of rapamycin (mTOR) pathways thus driving cell proliferation, angiogenesis, glycolysis and anti-apoptosis pathways leading to tumorigenesis and increased metastasis [31],[32],[33],[34],[35] [Figure 1]. The connection between hyperinsulinemia and oncogenesis, known as the insulin-cancer hypothesis, was first proposed in the early 1990s. [36],[37] Obesity leads to insulin resistance, hyperinsulinemia and greater bioavailability of IGF-1. [12],[18],[38] IGF-1 exhibits effects similar to insulin because of their shared downstream signaling pathways and studies suggest that IGF-1 may be the more relevant obesity-mediated growth factor. [39] In the Women’s Health Initiative observational study, BC incidence was increased 2.4-fold in women in the highest quartile of fasting insulin concentrations compared with women in the lowest quartile. Ultimately, insulin/IGF-1 signaling may explain the relationship between BMI and BC risk, independent of estradiol levels. [40] Furthermore, the alterations in IGF-1 signaling and inflammation observed in animals that over-express insulin-like growth factor receptor (IGF-1R) result in the formation of mammary tumors that share features with BBC. [41] Conversely, genetic ablation of IGF-1R delays tumor onset as does treatment with an IGF-1R inhibitor. [42] Three classes of inhibitors targeting the IGF-1R pathway are currently in clinical trials including anti-receptor antibodies, anti-ligand antibodies and small molecule tyrosine kinase inhibitors. [38] First, anti-receptor antibodies are specific to IGF-1R and spare insulin receptors. [43] Their mechanism of action is through inhibition of the binding of ligands to the IGF-1R. [43] However, these antibodies are associated with severe side-effects including hyperglycemia and hyperinsulinemia. [43] Although, phase II clinical trials with these antibodies showed promise, early phase III clinical trials have demonstrated lack of efficacy and metabolic toxicity (hyperglycemia). [43] Second, anti-ligand antibodies are specific to IGF-1 and IGF-2 and do not cross react with insulin. [44] However, the concern regarding high levels of free insulin-like growth factor binding protein that normally bind to the IGFs is still being addressed. Third, tyrosine kinase inhibitors were developed to be specific to IGF-1R but tend to inhibit all members of the pathway including insulin. [45] Interestingly, these agents are proving to be safer than previously expected and may be of therapeutic advantage due to their broader range of inhibition. [45] In sum, although there was a strong rationale for targeting the IGF-1R pathway based on preclinical studies, clinical trials have so far not been proven to be useful in the treatment of cancer.
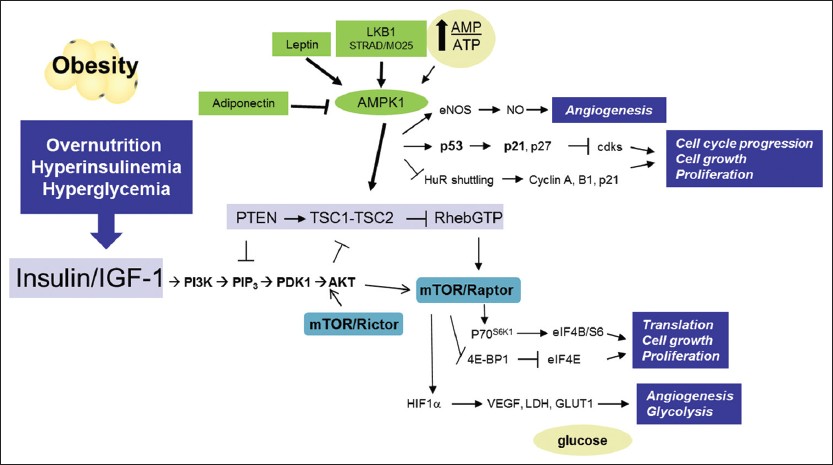 |
Figure 1: Obesity mediated alterations in nutrient sensitive pathways. Overnutrition in obesity leads to an increase in insulin and glucose. Insulin, insulin-like growth factor-1 and downstream pathways are activated increasing fuel metabolism and cell growth through Akt-dependent pathways. Energy sensing pathways such as AMP-activated protein kinase and mammalian target of rapamycin are potential targets to blunt cell growth either through caloric restriction or pharmacologically. Click here to view |
Nutrient sensitive pathways, metabolism and cancer
There is great interest in controlling tumor growth through metabolic reprogramming. [46],[47] Glucose metabolism and growth control are tightly linked in proliferating cells and involve signaling pathways including the PI3K/Akt/mTOR pathway [48] [Figure 1]. The “Warburg effect” describes cells exhibiting a metabolic shift toward glycolysis, supporting increased production of biomass, especially amino acids and nucleic acids. [49] We found that BC subtypes could be characterized as more or less aggressive using metabolomics analysis, which measure Warburg-like changes within tumors. [50] Furthermore, fibroblasts isolated from patient BBC tumors and co-cultured with BBC-like epithelial cells drove glucose transporter-1 expression and glucose metabolism in BBC-like epithelial cells, which correlated with metabolic phenotypes in patient samples, [50] thus denoting integrated cross-talk between the stromal and epithelial phenotypes on tumor metabolism. In experimental animal models, diet-induced obesity leads to activation of Akt and mTOR in a variety of epithelial tissues. [31],[32] Conversely, calorie restriction represses signaling through the PI3K/Akt/mTOR pathway. [31],[32]
Recent evidence suggests that metformin, an anti-diabetic biguanide medication, lowers cancer risk and reduces cancer incidence and deaths among diabetic patients, hence clinical trials are underway, some focusing on BC, [51],[52],[53],[54] and reviewed in. [38] Metformin inhibits complex 1 of the mitochondrial electron transport chain and therefore, oxidative phosphorylation (i.e., adenosine triphosphate production). The subsequent low energy state drives 5′ Adenosine monophosphate (AMP)-activated protein kinase (AMPK) activity [Figure 1], a master metabolic regulator that modulates multiple anabolic and proliferation pathways, including the PI3K/Akt/mTOR pathway and glucose uptake. [47],[55],[56] Cancer incidence in diabetic patients on metformin was 7.3% compared with 11.6% in non-metformin users. [53] However, it remains unclear whether metformin’s potential anti-neoplastic effects relates to the systemic action of this drug, (e.g., by reducing circulating glucose and insulin levels) and/or some direct action on cancer cells. Preclinical data suggests that the anti-tumorigenic efficacy of metformin is dependent on the obese and insulin resistant state. [57],[58] Metformin-mediated AMPK activation decreases cell growth in vitro and in xenograft models through inhibition of mTOR. [59],[60] Thus, metformin may have dual interrelated anti-tumorigenic functions – inhibition of the mTOR pathway and disruption of glucose uptake by cancer cells. Taken together, obesity is a high-energy condition that promotes increased growth factor signaling through the insulin/IGF-1 axis and is a nutrient-rich environment ultimately driving excessive stimulation of the PI3K/Akt/mTOR pathway [Figure 1]. [34],[35],[61],[62]
Estrogens
Obesity can drive carcinogenesis by increasing estrogen concentrations. Obese adipose tissues up-regulate the conversion of androstenedione to estrone and testosterone to estradiol, [63],[64] while at the same time reducing sex hormone-binding globulin capacity which leads to increased levels of free, biologically active estrogens. [64],[65] In postmenopausal women, aromatization of androgens in the adipose tissue by aromatase elevates local and circulating levels of estrogen, although this is not true in some murine models (data not shown). Obesity-associated cytokines including interleukin-6 and tumor necrosis factor alpha (TNF-α), and adipokines, such as leptin, stimulate aromatase activity leading to an increase in estrogen synthesis, while weight loss has been shown to blunt estrogen levels. [64],[66] The role of obesity in regulation of estrogen and progesterone are reviewed in detail. [67],[68]
Adipokines
The adipose tissue secretes several growth factors and cytokines, known as the adipokines, involved in energy homeostasis, immunity, angiogenesis, and endocrine signaling. [18] Leptin is produced mainly by expanding white adipose tissue, and is involved in the regulation of energy homeostasis. [18] Leptin activates the Janus kinase (JAK)/signal transducer and activator of transcription (STAT), mitogen activated protein kinase (MAPK) -extracellular signal-regulated kinases (ERK1/2), PI3K/Akt, AMPK, and insulin receptor substrates pathways [Figure 1]. Leptin is mitogenic, anti-apoptotic, pro-angiogenic, and pro-inflammatory, and thus, is implicated in the stimulation, migration, and invasion of tumor cells, as well as in the production of cytokines by macrophages. [69] Leptin also induces activation of the ERBB-2 pathway which interacts with IGF-1 to promote migration and metastasis of tumor cells. [70],[71]
Adiponectin is inversely correlated with obesity and leptin concentrations. Adiponectin regulates energy intake and expenditure, and plays an anti-inflammatory, anti-atherogenic, and insulin sensitizing role in metabolism. In cancer, adiponectin acts as anti-angiogenic, antiproliferative, pro-apoptotic, and anti-inflammatory mediator through AMPK and peroxisome proliferator-activated receptor signaling [72] [Figure 1]. Adiponectin blocks induction of angiogenic vascular endothelial growth factor (VEGF) by suppressing TNF-α, inducing apoptosis and inhibiting migration in the vascular endothelial cells. [73],[74] Decreased adiponectin level correlates with increased BC risk in postmenopausal women [75],[76] and conversely high levels of adiponectin are associated with an increased BC survival. [77] The leptin: adiponectin ratio may be the most relevant indicator of cancer risk as reviewed in detail. [12],[78] VEGF, basic fibroblast growth factor, and hepatocyte growth factor are other growth factors involved in BC-related angiogenesis under investigation. [50],[79],[80]
Normal microenvironment
While basic science research has traditionally focused on understanding the contribution of genomic mutations within the cancerous epithelial cells, the characteristics of the tissue microenvironment are proposed to play an integral role in supporting the proliferation of cancer cells. [81] In order to proliferate and escape apoptotic control mechanisms, transformed epithelial cells must adapt to – and take advantage of the conditions within the microenvironment in which they reside. [82],[83] In the breast, stromal cells including, adipocytes, fibroblasts and macrophages and other immune cells play fundamental roles in normal mammary development as well as carcinogenesis. [84] Macrophage, eosinophil, and mast cell influx typify different developmental stages and aid in mammary gland formation and involution (remodeling) in the post-natal period, during puberty, after pregnancy and lactation. [22],[84],[85],[86],[87] Work by our group and others has increasingly linked obesity and inflammation in various adipose depots. [22],[88],[89] In non-breast tissue, macrophages infiltrate adipose tissue at the onset of weight gain and directly contribute to and perpetuate the chronic inflammation characteristic of obese adipose, which is a major causal factor of insulin resistance. [18],[90],[91] Our findings [22] , corroborated by those of Dr. Dannenberg’s research group have demonstrated that both obese women and murine models also have elevated macrophage infiltration in normal breast tissue.[92],[93]
Tumor microenvironment
Macrophage infiltration in the tumor microenvironment is also a well-established phenomenon; tumor associated macrophages (TAMs) correlate with increased tumor angiogenesis, positive lymph node status and reduced survival of BC patients. [94],[95],[96] Furthermore, macrophage influx during ductal involution is proposed to create the pro-inflammatory microenvironment that promotes pregnancy associated BC. [84] Macrophage infiltration in the microenvironment of ductal carcinoma in situ is associated with high-grade, ER- and PR-negative BCs. [97] Indeed, BBC is characterized by unique epithelial-stromal interactions, [50],[98] relative to other BC subtypes, [98] which likely play a role in its etiology. TAM production of tumor-promoting factors, such as epidermal growth factor (EGF) [96] and VEGF, [94] are recognized as particularly important in BBC, [99],[100] and are microenvironment-mediated mechanisms of BBC onset. Other stromal cells also contribute to alterations in the tumor microenvironment. Dr. Lisanti’s research group have shown that stroma plays a vital role in the metabolism of the tumor, termed the “reverse Warburg effect,” via fibroblast-mediated metabolism. [101],[102],[103],[104] Cancer associated fibroblasts react to oxidative stress emitted from tumor cells by driving the production of inflammatory mediators and up-regulating glycolysis in a hypoxia-inducible factor α/Nuclear Factor -κB-dependent manner to generate metabolites for energy and proliferation of neighboring epithelial tumor cells. [101],[105] This cross-talk allows for tumor progression that is intricately linked to stromal metabolism. Finally, Stewart et al. have demonstrated that basal-like epithelial cells foster a pro-inflammatory milieu that drives differentiation and polarization of monocytes to macrophages [23],[98] and is established by direct interactions between primary BBC patient-derived fibroblasts and mammary epithelial cells in culture. [50] Recent studies have also identified a role for neutrophils and mast cells in promoting a pro-tumorigenic breast microenvironment by promoting the release of cytokines and chemokines, reactive oxygen and proteases. [106]
Anti-inflammatory approaches
Will addressing inflammation cure cancer? Although a tantalizing hypothesis, this is not a simple question to answer due to the complicated nature of macrophage polarization. Macrophages polarized toward the M1-like or the classical phenotype, tend to be pro-tumoricidal while M2-like or alternatively activated macrophages, may be protective of tumor cell growth [107] [Figure 2]. In BC, T-helper 2 cell-derived IL-4 mediates M2 polarization and promotes metastasis. [108] B cells in the microenvironment can also skew macrophage function and promote tumor progression via IL-10 induction. [108],[109] Animal models of BC have demonstrated that inflammatory processes contribute to tumor proliferation and metastasis while anti-inflammatory drugs are chemopreventive. [87],[94],[96],[110],[111],[112],[113],[114],[115] Epidemiological studies indicate that anti-inflammatory drugs reduce the risk of both receptor-positive and -negative BC. [29],[116],[117] Interactions between the stromal and metabolic microenvironment likely regulate the immune cell population, TAM infiltration and polarization and microenvironment-mediated plasticity; all which are currently under investigation. Understanding how metabolic pathways are altered in tumors and how cancer cells benefit from tumor-specific metabolic changes may contribute to the identification of novel therapeutic targets and the development of more effective cancer therapies.
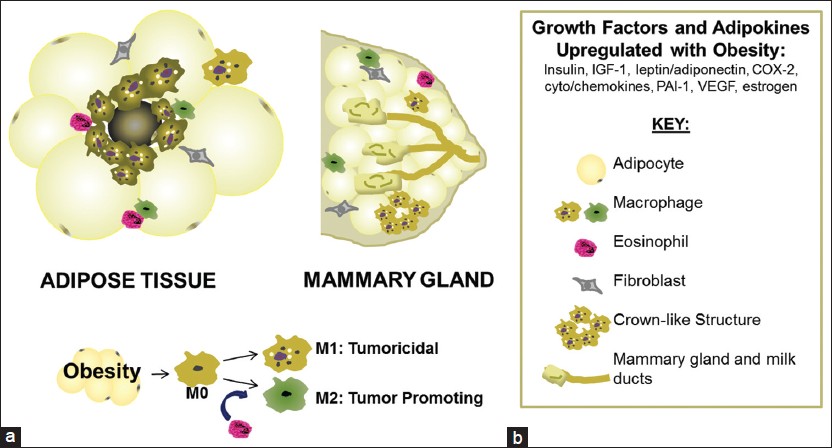 |
Figure 2: Recruitment of inflammatory cells in the tumor microenvironment mediated by obesity. (a) Obese adipose tissue is characterized by hypertrophy and hyperplasia of adipocytes, apoptosis and a shift in the stroma from less inflammatory eosinophils and M2-polarized cells (promoting insulin sensitivity), to an environment rich in pro-inflammatory M1-polarized macrophages, crown-like structures and activated fibroblasts. The mammary gland displays many of the same phenotypic changes with obesity. This low-level chronic inflammation in the tissue is known to induce oncogenesis, with an interesting shift in polarized macrophages from less M1 tumoricidal macrophages to more M2 tumor-promoting macrophages (b). Click here to view |
Conclusion
While some epidemiologic studies fail to find evidence supporting diet-mediated risk on BC, [118],[119],[120] lifestyle factors such as geographic differences and immigrant studies as well as rodent models, suggest that diet-induced obesity may play a role in oncogenesis. The underlying causes of obesity represent a complex web of interactions including inherited genetic traits, low physical activity levels, environmental factors such as toxins and access to affordable, healthy food, cultural identity, socioeconomic status and others. [121] Despite all of the work aimed at ameliorating obesity, recent projections have estimated that 51% of the US population will be obese by 2030. [122] Because obesity rates continue to increase worldwide, understanding the role of obesity in carcinogenesis is a question with high public health impact, with the added potential of reducing health disparities associated with certain cancers. Prevention of BC via reduction of obesity offers an important and unique opportunity for intervention. Nearly 90,000 cancer related deaths in the US could be avoided if adults maintained a BMI < 25 for life. [123]
Effective and targeted prevention of cancer among obese individuals depends upon understanding the molecular underpinnings of obesity-associated BC risk. Specifically, researchers need to elucidate the effects of aberrant systemic metabolism on the characteristics of tissue microenvironments and the promotion of cancer. Highlighting the relationship between nutritional state and disease, populations that suffered from severe caloric restriction, such as during World War II WWII and other famines, exhibit lower death rates from a broad spectrum of cancers. [124],[125] Epidemiologic associations suggest that caloric restriction may be protective against BBC, [27] but this needs to be validated in additional animal studies and clinical trials. Caloric restriction or drugs that mimic it (like mTOR inhibitors) are approaches currently under study in primate models [126],[127],[128] and rodents (reviewed in detail [125] ). In addition, the glucose lowering agent metformin has been effective in reducing overall cancer incidence and mortality. [129],[130] Ideally, integration of tumor characteristics and the microenvironment using physiologic, immunohistologic, metabolomic, and transcriptomic data should be used to construct a complete picture of the role of obesity-mediated alterations in the etiology of cancer. Furthermore, focusing on a specific window of susceptibility to cancer onset or metabolic state may be the most direct approach to understand links between obesity and oncogenesis.
Acknowledgments
We thank the Makowski Lab for critical review of this manuscript. This work is supported in part by National Institutes of Health (NIH) grant P30DK056350 to the University of North Carolina at Chapel Hill Nutrition Obesity Research Center. This publication was made possible by the Breast Cancer and the Environment Research Program U01ES019472 from the National Institute of Environmental Health Sciences and the National Cancer Institute, NIH, Department of Health and Human Services. Liza Makowski is supported by the University of North Carolina Cancer Research Fund.
References
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.  [PUBMED] |
| 2. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38.  |
| 3. | WHO. Obesity and overweight. World Health Organization; WHO Media Center, Geneva, 2011.  |
| 4. | Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491-7.  [PUBMED] |
| 5. | Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 2012;307:483-90.  [PUBMED] |
| 6. | Klein JD, Dietz W. Childhood obesity: The new tobacco. Health Aff (Millwood) 2010;29:388-92.  [PUBMED] |
| 7. | Haslam SZ, Schwartz RC. Is there a link between a high-fat diet during puberty and breast cancer risk? Womens Health (Lond Engl) 2011;7:1-3.  [PUBMED] |
| 8. | Bliss JM, Gray R. Breast cancer and hormone-replacement therapy: The Million Women Study. Lancet 2003;362:1328-9.  |
| 9. | Marsden J, A’Hern R. The Million Women Study and breast cancer. J Br Menopause Soc 2003;9:95, 97.  [PUBMED] |
| 10. | Beral V, Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet 2003;362:419-27.  |
| 11. | Colditz GA, Wolin KY, Gehlert S. Applying what we know to accelerate cancer prevention. Sci Transl Med 2012;4:127rv4.  [PUBMED] |
| 12. | Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, et al. Obesity, energy balance, and cancer: New opportunities for prevention. Cancer Prev Res (Phila) 2012;5:1260-72.  [PUBMED] |
| 13. | Popkin BM. Contemporary nutritional transition: Determinants of diet and its impact on body composition. Proc Nutr Soc 2011;70:82-91.  [PUBMED] |
| 14. | Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70:3-21.  [PUBMED] |
| 15. | Adams KM, Kohlmeier M, Powell M, Zeisel SH. Nutrition in medicine: Nutrition education for medical students and residents. Nutr Clin Pract 2010;25:471-80.  [PUBMED] |
| 16. | Yang C, Tan YS, Harkema JR, Haslam SZ. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod Toxicol 2009;27:299-306.  [PUBMED] |
| 17. | Olson LK, Tan Y, Zhao Y, Aupperlee MD, Haslam SZ. Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness. Int J Obes (Lond) 2010;34:1415-26.  [PUBMED] |
| 18. | Johnson AR, Milner JJ, Makowski L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol Rev 2012;249:218-38.  [PUBMED] |
| 19. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29.  [PUBMED] |
| 20. | DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin 2011;61:409-18.  [PUBMED] |
| 21. | Sundaram S, Freemerman AJ, McNaughton KK, Galanko JA, Bendt KM, Darr DB, et al. Role of HGF in obesity-associated tumorigenesis: C3 (1)-Tag mice as a model for human basal-like breast cancer. Breast Cancre Res Treat 2013; Submitted.  |
| 22. | Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat 2012;131:1003-12.  [PUBMED] |
| 23. | Stewart DA, Yang Y, Makowski L, Troester MA. Basal-like breast cancer cells induce phenotypic and genomic changes in macrophages. Mol Cancer Res 2012;10:727-38.  [PUBMED] |
| 24. | Fan C, Oh DS, Wessels L, Weigelt B, Nuyten DS, Nobel AB, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med 2006;355:560-9.  [PUBMED] |
| 25. | Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61-70.  [PUBMED] |
| 26. | Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492-502.  [PUBMED] |
| 27. | Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 2008;109:123-39.  [PUBMED] |
| 28. | Toft DJ, Cryns VL. Minireview: Basal-like breast cancer: From molecular profiles to targeted therapies. Mol Endocrinol 2011;25:199-211.  [PUBMED] |
| 29. | Gordon RR, Hunter KW, La Merrill M, Sørensen P, Threadgill DW, Pomp D. Genotype X diet interactions in mice predisposed to mammary cancer: II. Tumors and metastasis. Mamm Genome 2008;19:179-89.  |
| 30. | Lam JB, Chow KH, Xu A, Lam KS, Liu J, Wong NS, et al. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS One 2009;4:e4968.  [PUBMED] |
| 31. | Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila) 2008;1:65-76.  [PUBMED] |
| 32. | Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res 2008;68:5492-9.  [PUBMED] |
| 33. | Moore T, Carbajal S, Beltran L, Perkins SN, Yakar S, Leroith D, et al. Reduced susceptibility to two-stage skin carcinogenesis in mice with low circulating insulin-like growth factor I levels. Cancer Res 2008;68:3680-8.  [PUBMED] |
| 34. | Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: At the crossroads of obesity, diabetes and cancer. Trends Mol Med 2007;13:252-9.  [PUBMED] |
| 35. | Hursting SD, Lashinger LM, Wheatley KW, Rogers CJ, Colbert LH, Nunez NP, et al. Reducing the weight of cancer: Mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract Res Clin Endocrinol Metab 2008;22:659-69.  [PUBMED] |
| 36. | Giovannucci E. Insulin and colon cancer. Cancer Causes Control 1995;6:164-79.  [PUBMED] |
| 37. | McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: Are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev 1994;3:687-95.  [PUBMED] |
| 38. | Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat Rev Cancer 2012;12:159-69.  [PUBMED] |
| 39. | Lashinger LM, Malone LM, McArthur MJ, Goldberg JA, Daniels EA, Pavone A, et al. Genetic reduction of insulin-like growth factor-1 mimics the anticancer effects of calorie restriction on cyclooxygenase-2-driven pancreatic neoplasia. Cancer Prev Res (Phila) 2011;4:1030-40.  [PUBMED] |
| 40. | Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 2009;101:48-60.  [PUBMED] |
| 41. | van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev 2009;18:2569-78.  [PUBMED] |
| 42. | Klinakis A, Szabolcs M, Chen G, Xuan S, Hibshoosh H, Efstratiadis A. Igf1r as a therapeutic target in a mouse model of basal-like breast cancer. Proc Natl Acad Sci U S A 2009;106:2359-64.  [PUBMED] |
| 43. | Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: Early clinical trial results and future directions. Oncogene 2009;28:3009-21.  [PUBMED] |
| 44. | Gao J, Chesebrough JW, Cartlidge SA, Ricketts SA, Incognito L, Veldman-Jones M, et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res 2011;71:1029-40.  [PUBMED] |
| 45. | García-Echeverría C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell 2004;5:231-9.  |
| 46. | Ward PS, Thompson CB. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012;21:297-308.  [PUBMED] |
| 47. | Makowski L, Hayes DN. Role of LKB1 in lung cancer development. Br J Cancer 2008;99:683-8.  [PUBMED] |
| 48. | Yecies JL, Manning BD. mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med (Berl) 2011;89:221-8.  [PUBMED] |
| 49. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009;324:1029-33.  [PUBMED] |
| 50. | Brauer HA, Makowski L, Hoadley KA, Casbas-Hernandez P, Lang LJ, Romàn-Pèrez E, et al. Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer. Clin Cancer Res 2013;19:571-85.  |
| 51. | Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304-5.  [PUBMED] |
| 52. | Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006;29:254-8.  [PUBMED] |
| 53. | Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620-5.  |
| 54. | Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009;27:3297-302.  [PUBMED] |
| 55. | Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: A common mechanism contributing to their antidiabetic actions? Diabetes 2004;53:1052-9.  [PUBMED] |
| 56. | Hadad SM, Fleming S, Thompson AM. Targeting AMPK: A new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol 2008;67:1-7.  [PUBMED] |
| 57. | Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer 2008;15:833-9.  [PUBMED] |
| 58. | Phoenix KN, Vumbaca F, Fox MM, Evans R, Claffey KP. Dietary energy availability affects primary and metastatic breast cancer and metformin efficacy. Breast Cancer Res Treat 2010;123:333-44.  [PUBMED] |
| 59. | Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006;66:10269-73.  [PUBMED] |
| 60. | Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 2009;69:7507-11.  [PUBMED] |
| 61. | Wysocki PJ, Wierusz-Wysocka B. Obesity, hyperinsulinemia and breast cancer: Novel targets and a novel role for metformin. Expert Rev Mol Diagn 2010;10:509-19.  [PUBMED] |
| 62. | Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: A therapeutic opportunity in breast cancer. Clin Cancer Res 2010;16:1695-700.  |
| 63. | Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol Biomarkers Prev 2002;11:1531-43.  [PUBMED] |
| 64. | Imayama I, Mason C, Duggan C. Mechanisms linking obesity to cancer risk. In: McTiernan A, editor. Physical Activity, Dietary Calorie Restriction, and Cancer. Vol. 3. New York: Springer; 2011. p. 99-142.  |
| 65. | Calle EE, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579-91.  [PUBMED] |
| 66. | McTiernan A, Tworoger SS, Rajan KB, Yasui Y, Sorenson B, Ulrich CM, et al. Effect of exercise on serum androgens in postmenopausal women: A 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2004;13:1099-105.  [PUBMED] |
| 67. | Hirschberg AL. Sex hormones, appetite and eating behaviour in women. Maturitas 2012;71:248-56.  [PUBMED] |
| 68. | Esfahlan RJ, Zarghami N, Esfahlan AJ, Mollazadeh M, Nejati K, Nasiri M. The Possible impact of obesity on androgen, progesterone and estrogen receptors (ERα and ERβ) Gene expression in breast cancer patients. Breast Cancer (Auckl) 2011;5:227-37.  [PUBMED] |
| 69. | Paz-Filho G, Lim EL, Wong ML, Licinio J. Associations between adipokines and obesity-related cancer. Front Biosci (Landmark Ed) 2011;16:1634-50.  [PUBMED] |
| 70. | Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O’Regan RM, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res 2008;68:9712-22.  [PUBMED] |
| 71. | Eisenberg A, Biener E, Charlier M, Krishnan RV, Djiane J, Herman B, et al. Transactivation of erbB2 by short and long isoforms of leptin receptors. FEBS Lett 2004;565:139-42.  [PUBMED] |
| 72. | Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: A review of current evidence. Endocr Rev 2012;33:547-94.  [PUBMED] |
| 73. | Mahadev K, Wu X, Donnelly S, Ouedraogo R, Eckhart AD, Goldstein BJ. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc Res 2008;78:376-84.  |
| 74. | Hu D, Fukuhara A, Miyata Y, Yokoyama C, Otsuki M, Kihara S, et al. Adiponectin regulates vascular endothelial growth factor-C expression in macrophages via Syk-ERK pathway. PLoS One 2013;8:e56071.  [PUBMED] |
| 75. | Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, Alomeroglu M. Tissue levels of adiponectin in breast cancer patients. Med Oncol 2007;24:361-6.  [PUBMED] |
| 76. | Alokail MS, Al-Daghri N, Abdulkareem A, Draz HM, Yakout SM, Alnaami AM, et al. Metabolic syndrome biomarkers and early breast cancer in Saudi women: Evidence for the presence of a systemic stress response and/or a pre-existing metabolic syndrome-related neoplasia risk? BMC Cancer 2013;13:54.  [PUBMED] |
| 77. | Fabian CJ. Adiponectin: A risk biomarker and attractive target for chemoprevention. J Clin Oncol 2012;30:124-6.  |
| 78. | Grossmann ME, Cleary MP. The balance between leptin and adiponectin in the control of carcinogenesis-focus on mammary tumorigenesis. Biochimie 2012;94:2164-71.  [PUBMED] |
| 79. | Goldfarb M. The fibroblast growth factor family. Cell Growth Differ 1990;1:439-45.  [PUBMED] |
| 80. | Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 1995;333:1757-63.  [PUBMED] |
| 81. | Gatenby RA, Gillies RJ, Brown JS. Of cancer and cave fish. Nat Rev Cancer 2011;11:237-8.  [PUBMED] |
| 82. | Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med 2008;49 Suppl 2:24S-42S.  [PUBMED] |
| 83. | Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011;17:320-9.  [PUBMED] |
| 84. | O›Brien J, Schedin P. Macrophages in breast cancer: Do involution macrophages account for the poor prognosis of pregnancy-associated breast cancer? J Mammary Gland Biol Neoplasia 2009;14:145-57.  |
| 85. | Radisky DC, Hartmann LC. Mammary involution and breast cancer risk: Transgenic models and clinical studies. J Mammary Gland Biol Neoplasia 2009;14:181-91.  [PUBMED] |
| 86. | Maller O, Martinson H, Schedin P. Extracellular matrix composition reveals complex and dynamic stromal-epithelial interactions in the mammary gland. J Mammary Gland Biol Neoplasia 2010;15:301-18.  [PUBMED] |
| 87. | Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development 2000;127:2269-82.  [PUBMED] |
| 88. | Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity (Silver Spring) 2011;19:1109-17.  [PUBMED] |
| 89. | Sampey BP, Freemerman AJ, Zhang J, Kuan PF, Galanko JA, O’Connell TM, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS One 2012;7:e38812.  [PUBMED] |
| 90. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796-808.  [PUBMED] |
| 91. | Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821-30.  [PUBMED] |
| 92. | Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329-46.  [PUBMED] |
| 93. | Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021-9.  [PUBMED] |
| 94. | Leek RD, Hunt NC, Landers RJ, Lewis CE, Royds JA, Harris AL. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J Pathol 2000;190:430-6.  [PUBMED] |
| 95. | Aggarwal BB, Gehlot P. Inflammation and cancer: How friendly is the relationship for cancer patients? Curr Opin Pharmacol 2009;9:351-69.  [PUBMED] |
| 96. | O’Sullivan C, Lewis CE, Harris AL, McGee JO. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet 1993;342:148-9.  [PUBMED] |
| 97. | Sharma M, Beck AH, Webster JA, Espinosa I, Montgomery K, Varma S, et al. Analysis of stromal signatures in the tumor microenvironment of ductal carcinoma in situ. Breast Cancer Res Treat 2010;123:397-404.  [PUBMED] |
| 98. | Camp JT, Elloumi F, Roman-Perez E, Rein J, Stewart DA, Harrell JC, et al. Interactions with fibroblasts are distinct in Basal-like and luminal breast cancers. Mol Cancer Res 2011;9:3-13.  [PUBMED] |
| 99. | Hoadley KA, Weigman VJ, Fan C, Sawyer LR, He X, Troester MA, et al. EGFR associated expression profiles vary with breast tumor subtype. BMC Genomics 2007;8:258.  [PUBMED] |
| 100. | Anders C, Carey LA. Understanding and treating triple-negative breast cancer. Oncology (Williston Park) 2008;22:1233-9.  [PUBMED] |
| 101. | Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 2010;9:3256-76.  [PUBMED] |
| 102. | Migneco G, Whitaker-Menezes D, Chiavarina B, Castello-Cros R, Pavlides S, Pestell RG, et al. Glycolytic cancer associated fibroblasts promote breast cancer tumor growth, without a measurable increase in angiogenesis: Evidence for stromal-epithelial metabolic coupling. Cell Cycle 2010;9:2412-22.  |
| 103. | Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, et al. Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: Similarities with oxidative stress, inflammation, Alzheimer’s disease, and “Neuron-Glia Metabolic Coupling”. Aging (Albany NY) 2010;2:185-99.  [PUBMED] |
| 104. | Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009;8:3984-4001.  [PUBMED] |
| 105. | Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang C, Pavlides S, et al. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: Implications for PET imaging of human tumors. Cell Cycle 2011;10:2504-20.  [PUBMED] |
| 106. | Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7.  [PUBMED] |
| 107. | Stout RD, Watkins SK, Suttles J. Functional plasticity of macrophages: In situ reprogramming of tumor-associated macrophages. J Leukoc Biol 2009;86:1105-9.  [PUBMED] |
| 108. | Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787-95.  [PUBMED] |
| 109. | Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012;2012:948098.  |
| 110. | Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 2009;4:e6562.  [PUBMED] |
| 111. | Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 2007;67:2649-56.  [PUBMED] |
| 112. | Orendas P, Kassayova M, Kajo K, Ahlers I, Kubatka P, Bojkova B, et al. Celecoxib and melatonin in prevention of female rat mammary carcinogenesis. Neoplasma 2009;56:252-8.  [PUBMED] |
| 113. | Steele VE, Rao CV, Zhang Y, Patlolla J, Boring D, Kopelovich L, et al. Chemopreventive efficacy of naproxen and nitric oxide-naproxen in rodent models of colon, urinary bladder, and mammary cancers. Cancer Prev Res (Phila) 2009;2:951-6.  [PUBMED] |
| 114. | Woditschka S, Haag JD, Mau B, Lubet RA, Gould MN. Chemopreventive effects of celecoxib are limited to hormonally responsive mammary carcinomas in the neu-induced retroviral rat model. Breast Cancer Res 2008;10:R18.  [PUBMED] |
| 115. | Pan Q, Bao LW, Merajver SD. Tetrathiomolybdate inhibits angiogenesis and metastasis through suppression of the NFkappaB signaling cascade. Mol Cancer Res 2003;1:701-6.  [PUBMED] |
| 116. | Kirsh VA, Kreiger N, Cotterchio M, Sloan M, Theis B. Nonsteroidal antiinflammatory drug use and breast cancer risk: Subgroup findings. Am J Epidemiol 2007;166:709-16.  [PUBMED] |
| 117. | Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. Use of nonsteroidal antiinflammatory drugs and risk of breast cancer: The case-control surveillance Study revisited. Am J Epidemiol 2005;162:165-70.  [PUBMED] |
| 118. | Blackburn GL, Wang KA. Dietary fat reduction and breast cancer outcome: Results from the Women’s Intervention Nutrition Study (WINS). Am J Clin Nutr 2007;86:s878-81.  [PUBMED] |
| 119. | Pierce JP. Diet and breast cancer prognosis: Making sense of the women’s Healthy eating and living and women’s intervention nutrition study trials. Curr Opin Obstet Gynecol 2009;21:86-91.  [PUBMED] |
| 120. | Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: The Women’s Healthy Eating and Living (WHEL) Study. Control Clin Trials 2002;23:728-56.  [PUBMED] |
| 121. | Moller DE, Kaufman KD. Metabolic syndrome: A clinical and molecular perspective. Annu Rev Med 2005;56:45-62.  [PUBMED] |
| 122. | Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med 2012;42:563-70.  [PUBMED] |
| 123. | Gottlieb RJ. Obesity and cancer. J Lanc Gen Hosp 2009;4:143-5.  |
| 124. | Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol 2010;28:4058-65.  [PUBMED] |
| 125. | Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: Lessons learned from 30 years of calorie restriction research. Carcinogenesis 2010;31:83-9.  [PUBMED] |
| 126. | Willette AA, Bendlin BB, Colman RJ, Kastman EK, Field AS, Alexander AL, et al. Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes 2012;61:1036-42.  [PUBMED] |
| 127. | Willette AA, Gallagher C, Bendlin BB, McLaren DG, Kastman EK, Canu E, et al. Homocysteine, neural atrophy, and the effect of caloric restriction in rhesus monkeys. Neurobiol Aging 2012;33:670-80.  |
| 128. | Imayama I, Ulrich CM, Alfano CM, Wang C, Xiao L, Wener MH, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: A randomized controlled trial. Cancer Res 2012;72:2314-26.  [PUBMED] |
| 129. | Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: Translational challenges. J Mol Endocrinol 2012;48:R31-43.  [PUBMED] |
| 130. | Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: An International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol 2012;30:19-26.  [PUBMED] |
Authors
Dr. Sneha Sundaram: Department of Nutrition, Gillings School of Global Public Health and School of Medicine, UNC Chapel Hill, Chapel Hill, NC.
Dr. Amy R Johnson: Department of Nutrition, Gillings School of Global Public Health and School of Medicine, UNC Chapel Hill, Chapel Hill, NC.
Dr. Liza Makowski: Department of Nutrition, Gillings School of Global Public Health and School of Medicine, UNC Chapel Hill, Chapel Hill, NC.
